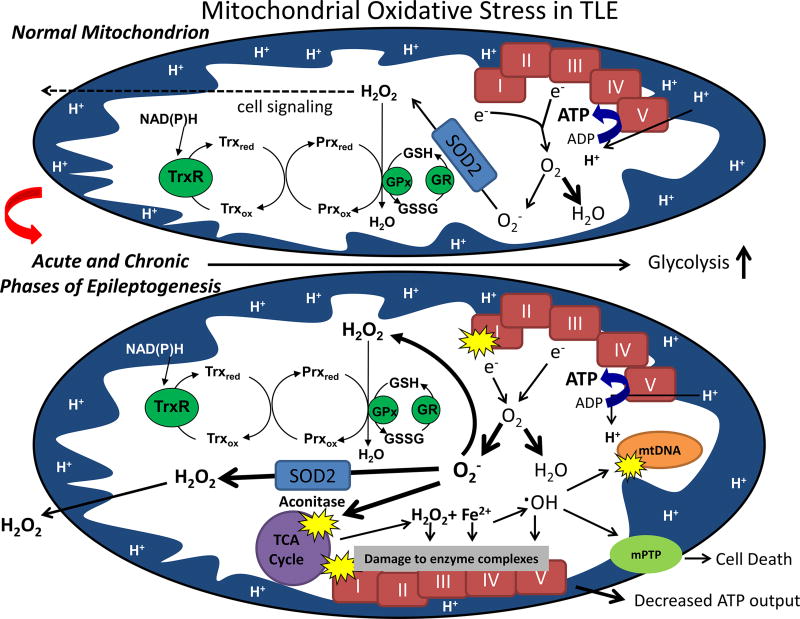
Figure 2.
Normal mitochondrial functions (depicted above) can be impaired following acute injury and during the chronic phase of epileptogenesis (depicted below). In normal brain mitochondria, superoxide (O2−.) produced via electron transport chain (ETC) Complexes I (CI) and III is detoxified by manganese superoxide dismutase (SOD2) while the majority of oxygen consumed is reduced to water; H2O2 resulting from enzymatic and spontaneous dismutation is detoxified via the glutathione and thioredoxin/peroxiredoxin (Trx/Prx) pathways such that low levels emitted from mitochondria play a redox signaling role. Following an inciting injury such as status epilepticus or trauma, steady-state levels of mitochondrial (O2− and H2O2) are elevated under conditions of low ATP production and inhibited CI[2, 25]. Inhibition of CI and aconitase are among two mechanisms that provide a feed-forward mechanism of reactive oxygen species (ROS)-induced ROS production by increasing O2− and release of redox-active iron and H2O2, respectively [34,86]. Oxidative damage to vicinal targets such as mtDNA, lipids and proteins ensues[53]. Higher levels of H2O2 emitted from mitochondria may damage plasmalemmal synaptic targets such as glutamate transporters and/or the ion channels potentially affecting neuronal excitability and cell death. mPTP=mitochondrial permeability transition pore; GSH=glutathione; GSSG=glutathione disulfide; GR=glutathione reductase; GPx=glutathione peroxidase.