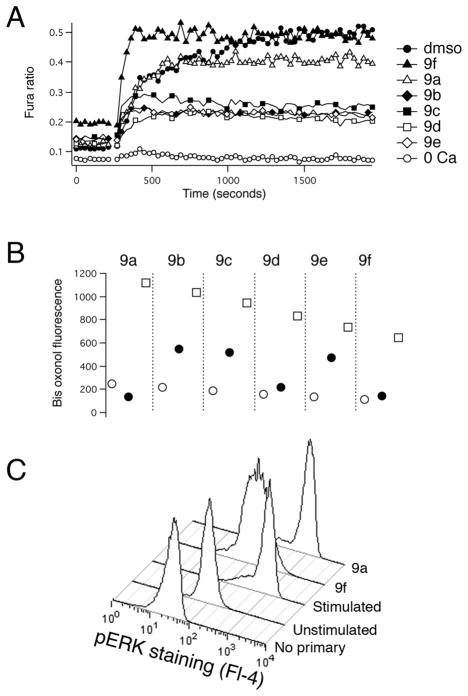
Figure 3. Assessing the mechanism of action of tetracyano-hexahydroisoindole compounds.
A) Representative Fura-2 ratio data for the six tetracyano-hexahydroisoindole compounds that inhibited lytic granule exocytosis by ~90% when tested at 10 μM. TG was added as indicated to trigger Ca2+ influx. For some traces, some symbols have been omitted for clarity. The lines connecting the symbols are drawn through those missing points. B) Representative bisoxonol data from one experiment of three. The geometric mean of bisoxonol fluorescence (Fl-1) is plotted for compounds (filled circles), Normal Ringer’s (open circles) and K+ Ringer’s (open squares). C) Cells treated as indicated were fixed, permeabilized and then stained with an anti-phospho ERK antibody followed by a CY5-labeled secondary antibody.