Figure 1.
Characterization of the Photoautotrophic Cell Suspension Culture of A. thaliana.
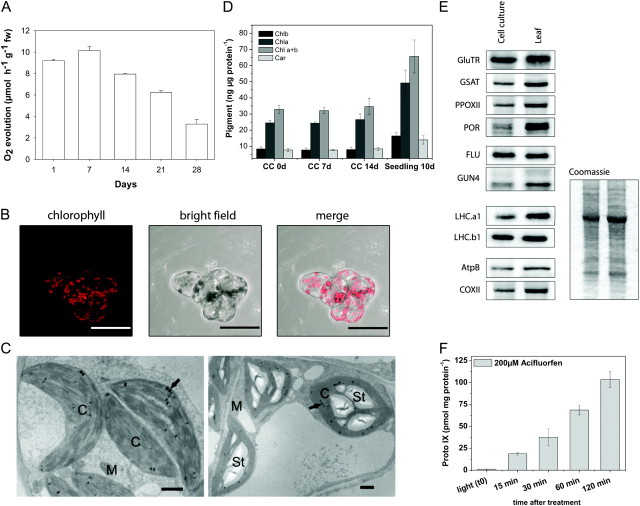
(A) Measurement of O2 evolution during growth.
(B) Analysis of PA cell culture by fluorescence and bright field light microscopy and merge of the pictures.
(C) Effect of a source–sink transition induced by glucose on starch accumulation in chloroplasts. Ultrastructure of untreated 2-week-old control PA A. thaliana suspension culture cells (left) and after treatment with 50 mM glucose for 48 h (right). Arrows, chloroplasts with plastoglobuli; M, mitochondria; St, starch grains; Bar = 1 μm.
(D) Quantitative analysis of chlorophylla (Chla), Chlorophyllb (Chlb), sum of Chla and Chlb (Chla+b), total carotenoids (Car) from the Arabidopsis PA cell culture (CC), and from 10-day-old soil-grown seedlings.
(E) Protein levels of PA CC and Arabidopsis seedlings in light. Immunodetected protein bands for the following proteins are shown: glutamyl-tRNA reductase (GluTR), glutamate 1-semialdehyde aminotransferase (GSAT), protoporphyrinogenoxidase II (PPOXII), protochlorophyl lideoxidoreductase (POR), fluorescent in blue light (FLU), genome uncoupled 4 (GUN4), light harvesting chlorophyll binding proteins (LHCa1 and LHCb1), beta subunit of ATP-synthase (AtpB), and cytochrome c oxidase II (COXII). Coomassie blue staining of the protein extract preparation used for immunodetection.
(F) Manipulation of tetrapyrrole synthesis by acifluorfen. Arabidopsis PA culture cells were incubated with 200 μM acifluorfen in light at approximately 180 μmol photons m−2 s−1. Compared to the steady-state level (t 0), a linear accumulation of Proto IX was detected over a period of 2 h.