Figure 8.
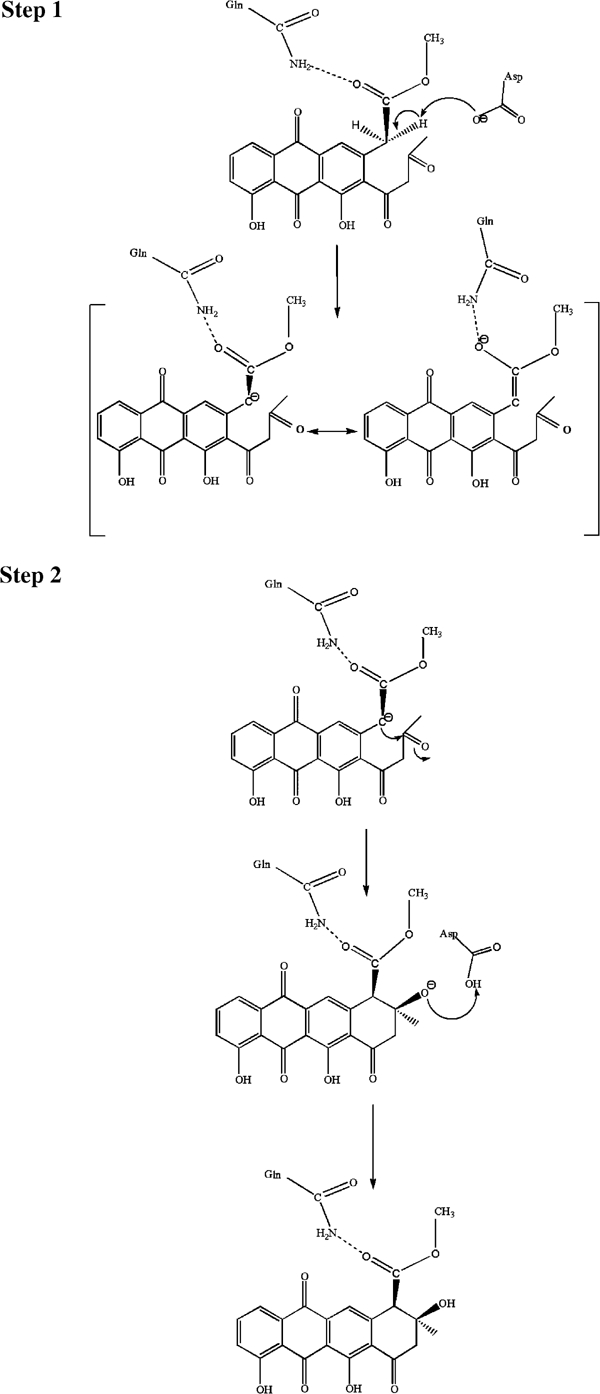
Proposed mechanism of the intramolecular aldol condensation catalysed by SnoaL. The first step of the reaction is the generation of the carbanion/enol(ate) intermediate by abstraction of a proton at carbon 10 by the conserved residue Asp121. The negative charge of the enol(ate) is stabilised by resonance over the aromatic ring system of the polyketide (not shown here). A minor stabilisation may be due to the hydrogen bond to the conserved residue Gln105. The carbanion then attacks the C9 carbonyl carbon atom resulting in ring closure. The negative charge at the carbonyl oxygen atom developing in the transition state is stabilised via a hydrogen bond to the protonated side chain of Asp121. The same residue also transfers a proton to the oxygen atom at C9 of the aglycone moiety.
