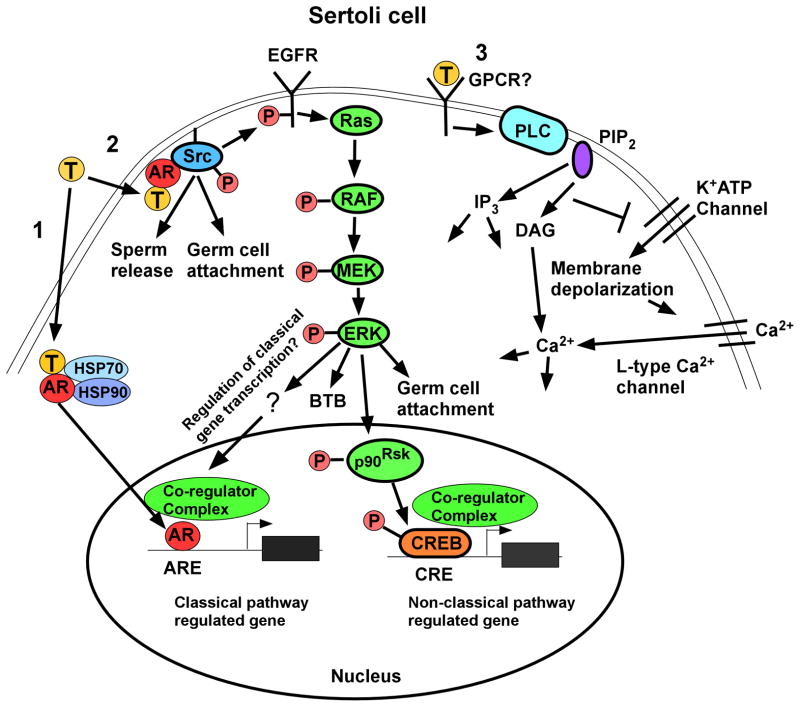
Figure 2.
Testosterone signaling pathways in Sertoli cells. Left (Pathway 1): The classical testosterone signaling pathway: Testosterone diffuses through the plasma membrane and binds with the AR that then undergoes an alteration in conformation allowing it to be released from heat shock proteins (HSP70, HSP90) in the cytoplasm. AR then translocates to the nucleus where it binds to specific DNA sequences called androgen response elements (AREs). AR binding to an ARE allows the recruitment of co-regulators (co-activator or co-repressor proteins) that alter the expression of genes and eventually cellular function. Middle (Pathway 2): The non-classical kinase activation pathway: Testosterone interacts with AR that is then able to bind with and activate Src, which is required for sperm release and germ cell attachment. Src also causes the activation of the EGF receptor that then activates the MAP kinase cascade most likely through Ras resulting in the sequential phosphorylation and activation of RAF and MEK and then ERK, which regulates BTB integrity and germ cell attachment. ERK may also regulate classical pathway-mediated gene expression through pathways that are not yet known for Sertoli cells. ERK also can facilitate non-classical pathway mediated gene expression. For example, ERK activates p90RSK kinase, which is known to phosphorylate CREB on serine 133. As a result, CREB-regulated genes can be induced by testosterone. Right (Pathway 3): The non-classical Ca2+ influx pathway: Testosterone interacts with a receptor in the plasma membrane that has characteristics of a Gq coupled G-protein coupled receptor (GPCR). Phospholipase C (PLC) is activated to cleave PIP2 into IP3 and DAG. Decreased PIP2 concentrations inhibit K+ATP channels causing membrane depolarization and Ca2+ entry via L-type Ca2+ channels.