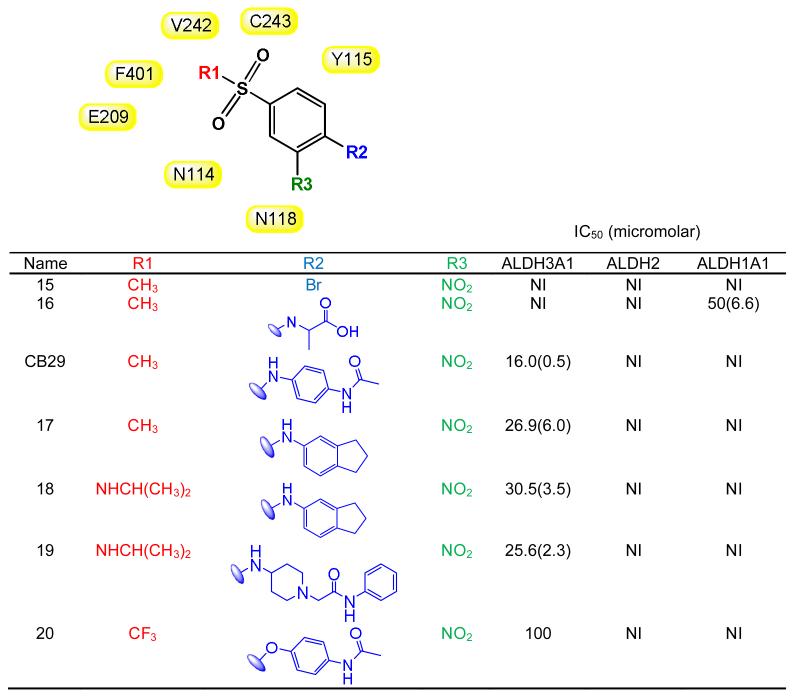
Table 3.
SAR for CB29-based compounds. (B) SAR showing the effect of first set of analogs that had substitutions other than aniline at R2 position (such as halogen, glycine and ethers). Two dimensional structures for the subgroups are generated using ChemBioDraw Ultra 12.0. Values in parentheses are S.D. for three independent assays. NI stands for no inhibition at 100 μM inhibitor concentration. Shaded in yellow are the residues of ALDH3A1 that are in close proximity with of CB29 at the respective positions. None of the listed analogs showed inhibition (<5%) to ALDH1A2, ALDH1A3 or ALDH1B1 up to 100 μM concentration.
|
SF767 cells also demonstrated robust ALDH3A1 expression, but lacked detectable expression of ALDH1A1 (Figure 3A & 3B). The antibodies utilized for detection of ALDH1A1 also detect ALDH1A2, ALDH1A3, ALDH1B1, and ALDH2 (Figure 3C), therefore SF767 appears devoid of all ALDH1 subtypes, as well as ALDH2. CCD-13Lu cells had no detectable expression of either ALDH1A1 or ALDH3A1 by immunoblot.
