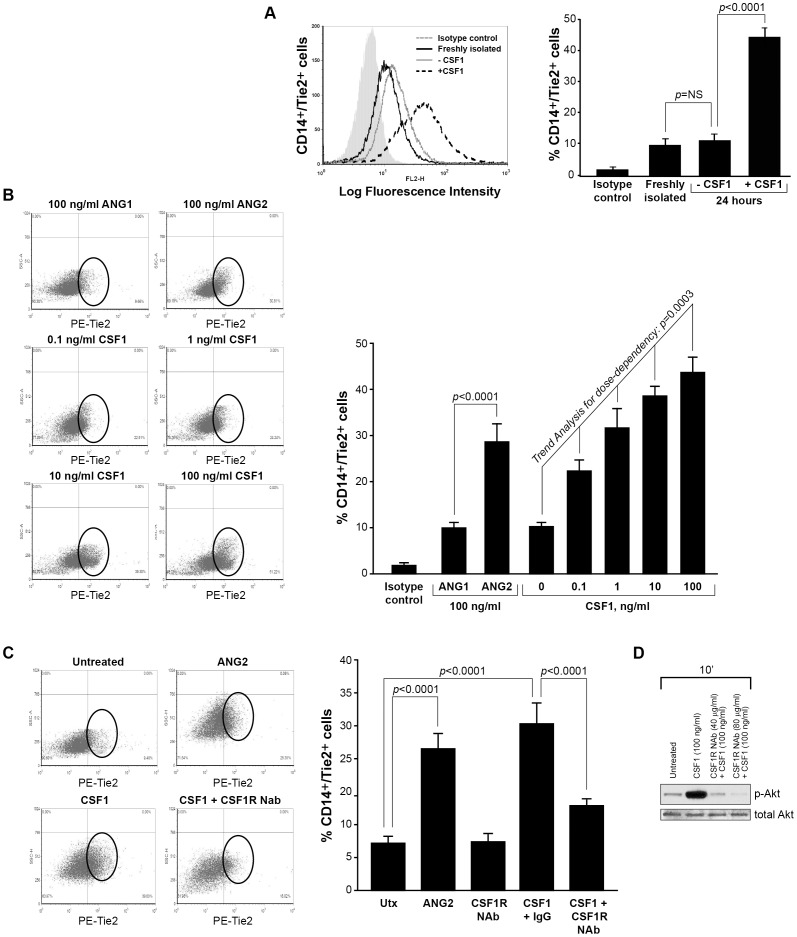
Figure 1. CSF1 up-regulates Tie2 receptor on CD14+ human monocytes.
(A) CD14+ monocytes were isolated from whole blood using CD14+ microbeads. Cells were fixed and immunostained using anti-human Tie2 receptor antibody or isotype control antibody immediately following isolation (Freshly isolated) or after treated without (-CSF1) or with rhCSF1 (100 ng/ml) (+CSF1) for 24 hours. N = 10 per group and results represent the mean ± SEM of Tie2-positivity. (B) CD14+ monocytes treated with rhANG1 (100 ng/ml), rhANG2 (100 ng/ml) or a dose-response of rhCSF1 (0, 0.1, 1, 10, 100 ng/ml). ANG2 up-regulated Tie2 expression compared to ANG1 and CSF1 induces a dose-escalation of Tie2 on CD14+ monocytes. N = 10 per group and results represent the mean ± SEM of Tie2-positivity. (C) CD14+ monocytes were left untreated (Utx) or treated with rhANG2 (100 ng/ml) (ANG2), rhCSF1 (100 ng/ml) (CSF1), CSF1R neutralizing antibody alone, or pre-treated with the CSF1R Nab for 30 minutes prior to stimulation with rhCSF1 (100 ng/ml) (CSF1R NAb+CSF1) for 24 hours. ANG2- and CSF1-treatment significantly increased Tie2 expression while the CSF1R NAb abrogated this effect. N = 8 per group and results represent the mean ± SEM of Tie2-positivity by flow cytometry. (D) CD14+ monocytes were left untreated (Untreated), pre-treated with CSF1R NAb (40 µg or 80 µg) for 30 minutes then treated with rhCSF1 (100 ng/ml) (CSF1R NAb+CSF1), or with rhCSF1 (100 ng/ml) alone (CSF1) for 10 minutes. Western blot analysis indicates that the CSF1R NAb was effective at reducing Akt1 phosphorylation.