Fig. 1.
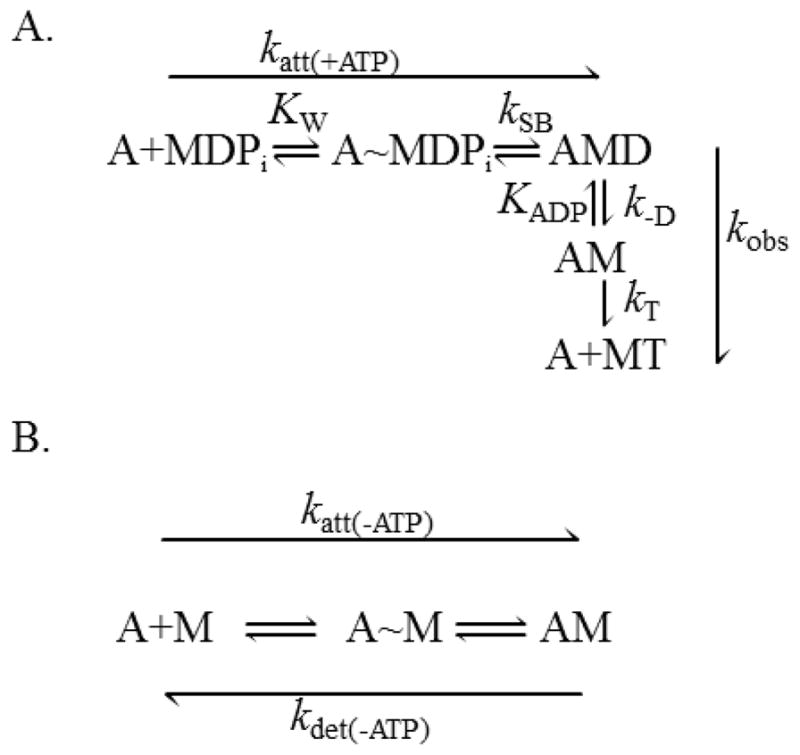
Kinetic schemes of strong A-M binding in the (A) presence and (B) absence of ATP. (A) In the presence of MgATP, A-M strong binding is a two-step process. Weak actin-myosin binding (A~MDPi) is thought to occur rapidly with an equilibrium binding constant KW. Strong A-M binding, with a rate constant kSB, is associated with Pi release and a myosin lever arm rotation. The effective rate constant for this two-step binding reaction is katt(+ATP) = KW·kSB. ADP release from A-M occurs with a rate constant k−D followed by ATP-induced A-M detachment with a second-order rate constant kT. (B) Even in the absence of ATP, A-M strong binding occurs through a two-step reaction with an effective rate constant, katt(−ATP). A-M detachment can occur spontaneously with a rate constant kdet(−ATP). A = actin, D = MgADP, T = MgATP, Pi = phosphate, M = myosin.
