FIGURE 3.
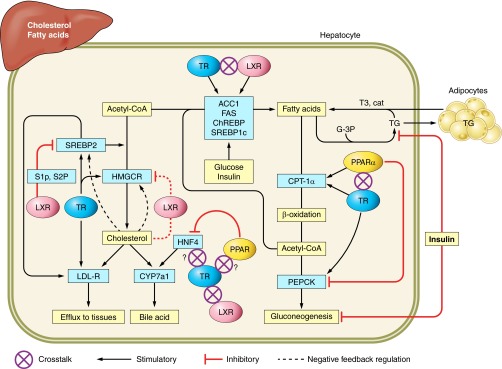
Lipid homeostasis in liver is coordinately regulated by direct actions of T3 and indirect crosstalk with nutrient-activated nuclear receptors. HMG-CoA reductase, a rate-limiting enzyme in cholesterol synthesis, and sterol response element binding protein (SREBP2) are stimulated by T3. HMG-CoA reductase is subject to feedback inhibition by cholesterol. The SREBP2 and LXR pathways respond to changes in cellular sterols. When cholesterol levels are low, SREBP2 is activated by LXR-mediated maturation by site 1 and site 2 proteases (S1P and S2P), then transported to the nucleus for activation of its target gene, HMG-CoA reductase. When cellular cholesterol is high, LXR inhibits S1P and S2P resulting in inactive SREBP2, which triggers sterol concentration-dependent HMGCR degradation. This then reduces cholesterol synthesis. CYP7a1 is a rate-limiting enzyme in bile acid synthesis. TR directly stimulates CYP7a1 gene expression in human liver. In mouse, both TR and LXR regulate CYP7a1 expression. Hepatocyte nuclear factor 4 (HNF4) also plays an important role in CYP7a1 gene expression. PPARγ reduces CYP7a1 gene expression by inhibiting HNF4 gene expression. Both TR and LXR play a role in fatty acid synthesis by regulating the expression of acetyl CoA carboxylase (ACC1), fatty acid synthase (FAS), carbohydrate response element binding protein (ChREBP), and SREBP1c. This regulation is mediated by similar DR4 response elements in these gene promoters. Fatty acid β-oxidation is controlled by the rate-limiting enzyme CPT-1α, which transports long-chain fatty acid into the mitochondria for oxidation. A functional TRE and PPRE are located in close proximity (50 bp apart) in the CPT-1α promoter. The mechanism of crosstalk between PPARα and TRα on the CTP-1α promoter has been previously characterized (151). Phosphoenolpyruvate carboxykinase (PEPCK) catalyzes the key step initiating gluconeogenesis and is regulated by hormones at the transcriptional level, including T3. In liver, PPARα ligand inhibits PEPCK mRNA expression. In adipocytes, PPARγ induces PEPCK expression to promote fat storage (not shown in figure). In the presence of glyceraldehyde-3-phosphate (G-3P), triglyceride is synthesized and transported to adipocytes. When energy is needed, there is central activation of the sympathetic nervous system and release of catecholamines, which acts on adipocytes to hydrolyze TG. T3 increases β-AR expression in adipocytes, which promotes catecholamine-induces lipolysis.
