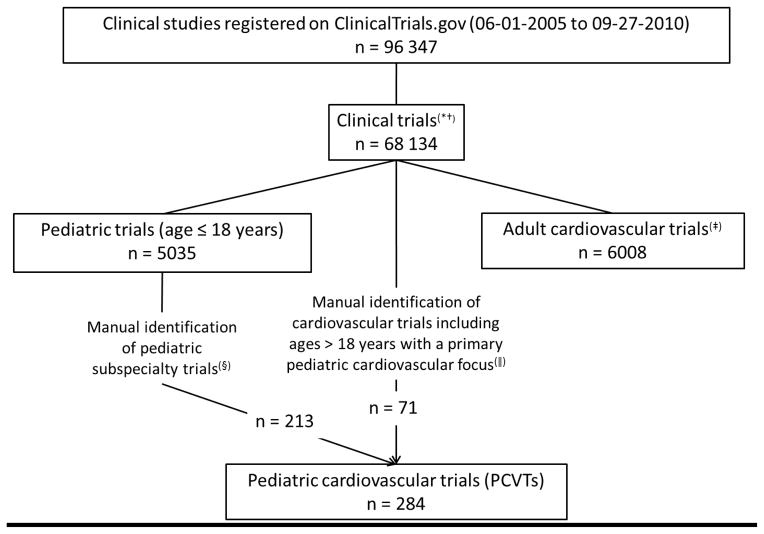
Figure I.
Consortium diagram: Inclusion criteria and methods for study selection.
*Clinical Trials refers to those trials registered as “interventional studies” on ClinicalTrials.gov. Interventional studies are defined by ClinicalTrials.gov as those in which an investigator assigns an intervention (including diagnostic, therapeutic or other types of interventions) based on a protocol.16
† Non-interventional or observational studies were excluded because there are no current registration requirements at ClinicalTrials.gov.
‡Methods for identification of adult cardiovascular trials described previously. These trials exclude trials enrolling participants < 18 years.15
§ Trials classified into pediatric subspecialties based on manual review of the trial characteristics as previously reported.11
||A pediatric cardiologist reviewed all adult cardiovascular trials that also enrolled subjects < 18 years of age and identified those that focused on pediatric cardiovascular diseases or conditions.