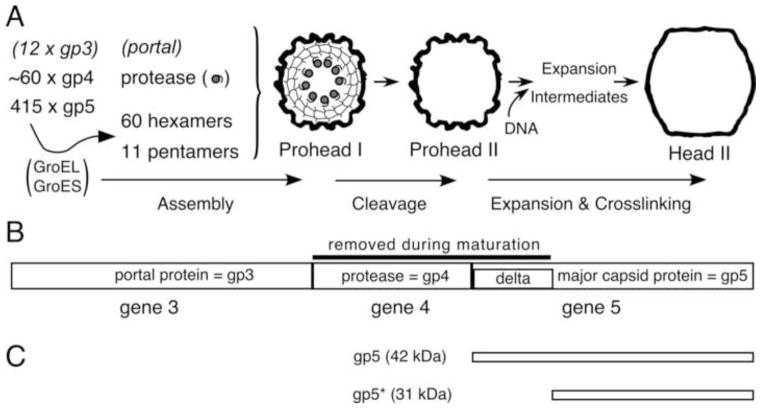
Fig. 1. HK97 Capsid Assembly Pathway.
The E. coli chaperonin proteins GroEL and GroES assist in the folding and assembly of the mcp subunit into hexamers and pentamers. These, collectively called capsomers, assemble, along with the portal and the protease, into Prohead I. The active protease, present inside the capsid, cleaves the delta domains, then cleaves itself. The resulting peptides escape from the capsid, leaving an empty Prohead II shell. DNA is packaged through the portal and induces the capsid to transform, via a series of expansion intermediates, into the mature Head II. Head II is stabilized by covalent crosslinks and is ready to attach a tail to form a viable phage. B. HK97 capsid assembly genes with protein names. The HK97 protease substrates, including the delta domain are identified. C. The major forms of the HK97 major capsid protein.