Photobody localization of Arabidopsis phytochrome B is tightly correlated with the degradation of a phytochrome-interacting factor and the inhibition of hypocotyl growth in the dark.
Abstract
Photobody localization of Arabidopsis (Arabidopsis thaliana) phytochrome B (phyB) fused to green fluorescent protein (PBG) correlates closely with the photoinhibition of hypocotyl elongation. However, the amino-terminal half of phyB fused to green fluorescent protein (NGB) is hypersensitive to light despite its inability to localize to photobodies. Therefore, the significance of photobodies in regulating hypocotyl growth remains debatable. Accumulating evidence indicates that under diurnal conditions, photoactivated phyB persists into darkness to inhibit hypocotyl elongation. Here, we examine whether photobodies are involved in inhibiting hypocotyl growth in darkness by comparing the PBG and NGB lines after the red light-to-dark transition. Surprisingly, after the transition from 10 μmol m−2 s−1 red light to darkness, PBG inhibits hypocotyl elongation three times longer than NGB. The disassembly of photobodies in PBG hypocotyl nuclei correlates tightly with the accumulation of the growth-promoting transcription factor PHYTOCHROME-INTERACTING FACTOR3 (PIF3). Destabilizing photobodies by either decreasing the light intensity or adding monochromatic far-red light treatment before the light-to-dark transition leads to faster PIF3 accumulation and a dramatic reduction in the capacity for hypocotyl growth inhibition in PBG. In contrast, NGB is defective in PIF3 degradation, and its hypocotyl growth in the dark is nearly unresponsive to changes in light conditions. Together, our results support the model that photobodies are required for the prolonged, light-dependent inhibition of hypocotyl elongation in the dark by repressing PIF3 accumulation and by stabilizing the far-red light-absorbing form of phyB. Our study suggests that photobody localization patterns of phyB could serve as instructive cues that control light-dependent photomorphogenetic responses in the dark.
Plant growth and development are extremely plastic in response to environmental light cues (Franklin and Quail, 2010; Kami et al., 2010). This light-dependent phenotypic plasticity is best exemplified by the photoinhibition of hypocotyl elongation during the seedling development of dicotyledonous plants, such as the reference plant species Arabidopsis (Arabidopsis thaliana). Plants perceive light through a number of photoreceptors, including the red light (R)- and far-red light (FR)-sensing phytochromes. Phytochromes are bilin-containing proteins that consist of two domains: an N-terminal photosensory/signaling domain and a C-terminal dimerization/localization domain (Rockwell et al., 2006; Nagatani, 2010). Because the phytochromobilin chromophore is buried inside the polypeptide moiety of the N-terminal domain, isomerization of the chromophore by R or FR absorption triggers photoconversion between two relatively stable phytochrome conformers: the inactive Pr and the active Pfr (Rockwell et al., 2006; Nagatani, 2010; Ulijasz and Vierstra, 2011). In addition to photoconversion between Pr and Pfr, Pfr is thermodynamically unstable and can spontaneously revert back to Pr in the dark in a process termed dark reversion (Furuya and Song, 1994; Nagy and Schäfer, 2002). Therefore, photoconversion and dark reversion together determine the equilibrium percentage of phytochrome in the active Pfr, which transmits signals to regulate downstream photomorphogenetic responses, such as the inhibition of hypocotyl growth. The Arabidopsis genome encodes five phytochrome genes, named phyA to phyE (Sharrock and Quail, 1989). Among the five phytochromes, phyB is the main phytochrome that mediates the perception of continuous R and changes in the R-to-FR ratio (Chen et al., 2004).
Phytochromes inhibit hypocotyl elongation by antagonizing a group of basic helix-loop-helix transcription factors, the phytochrome-interacting factors (PIFs; Leivar and Quail, 2011). Most PIFs, including PIF1, PIF3, PIF4, PIF5, and PIF7, promote hypocotyl growth (Huq and Quail, 2002; Fujimori et al., 2004; Huq et al., 2004; Khanna et al., 2004; Oh et al., 2004; Al-Sady et al., 2008; Lorrain et al., 2009; Li et al., 2012); the quadruple pif1pif3pif4pif5 (pifq) mutant exhibits reduced hypocotyl growth in the dark (Leivar et al., 2008, 2009; Shin et al., 2009). The current model suggests that phytochromes inhibit PIFs by at least two mechanisms. First, most PIF proteins are stable only in the dark; in the light, photoactivated phytochromes bind directly to PIFs and trigger their phosphorylation and subsequent degradation in the light (Al-Sady et al., 2006; Lorrain et al., 2008; Shen et al., 2008; Leivar and Quail, 2011; Ni et al., 2013). Second, phytochromes can inhibit the transcriptional activity of PIF1 and PIF3 by removing them from the promoters of their target genes (Park et al., 2012).
At the cellular level, one of the earliest responses to light is the translocation of photoactivated phytochromes from the cytoplasm to subnuclear foci called phytochrome speckles or photobodies (Yamaguchi et al., 1999; Kircher et al., 2002; Chen and Chory, 2011; Van Buskirk et al., 2012). Since the initial observation of photobodies 15 years ago (Yamaguchi et al., 1999), the necessity of photobodies in phytochrome signaling has been debated. Accumulating evidence supports the notion that photobody localization of phyB is required for the phytochrome-mediated inhibition of hypocotyl growth (Van Buskirk et al., 2012). For example, in continuous R, the steady-state photobody localization pattern (size and number) of phyB fused to GFP (PBG) is determined by the percentage of phyB in Pfr (Yamaguchi et al., 1999; Chen et al., 2003). Light conditions that shift the Pfr/Pr equilibrium in favor of Pfr promote the localization of phyB to large photobodies. Consistent with this notion, in high-intensity R, PBG localizes exclusively to a few large photobodies with diameters between 1 and 2 μm, and seedlings are correspondingly short (Chen et al., 2003, 2010). By contrast, in dim R or in light with a low R-to-FR ratio, PBG is localized to many small photobodies or is evenly dispersed in the nucleoplasm, and seedlings are taller (Chen et al., 2003). Together, these results support the idea that the localization of phyB to photobodies correlates tightly with the degree of hypocotyl growth inhibition (Chen et al., 2003).
Genetic analyses of mutants with abnormal phyB photobody morphology also support the correlation between the photobody localization of phyB and the inhibition of hypocotyl growth. Most loss-of-function phyB alleles are defective in phyB localization to large photobodies and are taller than the wild type (Kircher et al., 2002; Chen et al., 2003; Matsushita et al., 2003; Nito et al., 2013), whereas in gain-of-function phyB mutants, phyB localizes to large photobodies under dim light and hypocotyl elongation is more restricted than in the wild type (Ádám et al., 2011; Medzihradszky et al., 2013; Zhang et al., 2013). In the most extreme case, phyBY276H (YHB), a constitutively active phyB mutant, localizes to large photobodies regardless of light conditions and can inhibit hypocotyl growth even in the dark (Su and Lagarias, 2007). In the extragenic hemera (hmr) mutant, PBG fails to localize to large photobodies and localizes to small photobodies instead; hmr mutants have correspondingly longer hypocotyls than the wild type in R (Chen et al., 2010).
Although these results support the significance of photobodies in phytochrome signaling, one line of evidence stands out against this model. Studies of photobody localization using Arabidopsis phyB have shown that the C-terminal domain of phyB is involved in dimerization and is sufficient for both nuclear and photobody localization (Matsushita et al., 2003; Chen et al., 2005). When the C-terminal domain of phyB is replaced with a dimerization domain, a Simian Vacuolating Virus40 nuclear localization signal, and GFP, the chimeric protein, NGB (for the N-terminal half of phyB fused to GFP), does not localize to photobodies (Matsushita et al., 2003). However, NGB is hyperactive in inhibiting hypocotyl growth in the light, suggesting that photobodies are dispensable and might even play a negative role in the photoinhibition of hypocotyl elongation in the light (Matsushita et al., 2003; Palágyi et al., 2010).
To reconcile these contradictory conclusions about the roles of photobodies in phytochrome signaling and hypocotyl growth inhibition, a more detailed comparison between the PBG and NGB lines in hypocotyl growth regulation is warranted. In particular, recent studies show that, although plants perceive light during the day, under short days the maximum hypocotyl growth rate occurs at the end of the night (Nozue et al., 2007). Hypocotyl growth during the dark period is mediated by PIF3 as well as by PIF1, PIF4, and PIF5; the levels of PIFs are coincidentally regulated by the circadian clock and light (Nozue et al., 2007; Leivar et al., 2012a; Soy et al., 2012, 2014). The circadian clock mediates an increase in PIF4 and PIF5 gene expression at the end of the night (Nozue et al., 2007; Leivar et al., 2012a; Soy et al., 2012). In parallel, because of the slow dark reversion rate of phyB (Hennig et al., 1999; Rausenberger et al., 2010), photoactivated phyB persists into the night to inhibit the accumulation of PIFs (Nozue et al., 2007; Soy et al., 2012, 2014). Based on these recent results, it would be interesting to investigate whether photobodies are required for phyB-mediated hypocotyl growth inhibition and PIF stability in the dark.
Here, by comparing the kinetics of hypocotyl growth, PIF3 accumulation, and the expression of PIF target genes between the PBG and NGB lines following the red light-to-dark (R-to-D) transition, we show that PBG can repress hypocotyl growth for a prolonged period of time in the dark. In contrast, and contrary to its hyperactivity in hypocotyl growth inhibition in the light, NGB has substantially less capacity for repressing hypocotyl growth in the dark. We show a close correlation between the photobody localization of PBG, PIF3 degradation, and hypocotyl growth inhibition in the dark. Our results support the model that photobodies are required for the prolonged, light-dependent inhibition of hypocotyl elongation in the dark by stabilizing the Pfr of phyB and by repressing PIF3 accumulation.
RESULTS
PBG Represses Hypocotyl Growth for a Substantially Longer Period Than NGB in the Dark
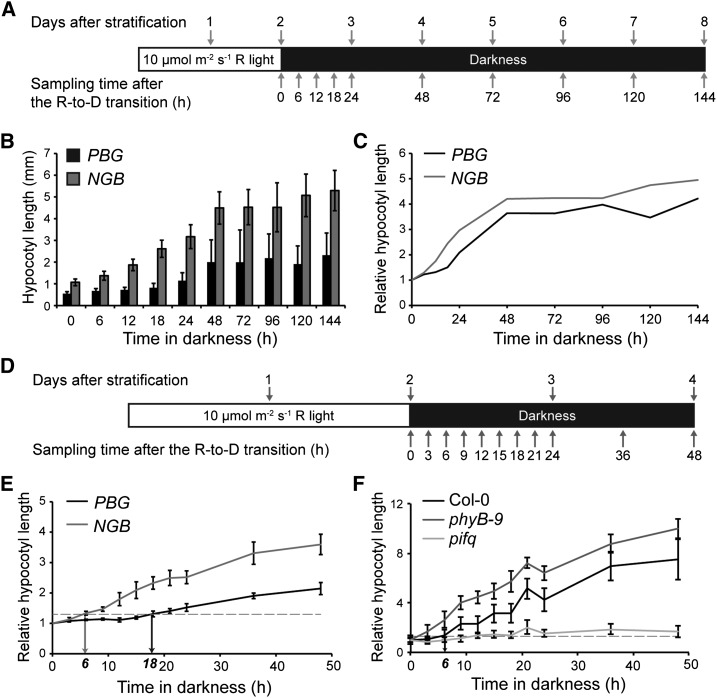
To examine whether photobodies are involved in phyB’s function in inhibiting hypocotyl growth in the dark, we wanted to design an R-to-D transition assay to measure the capacity of a seedling to inhibit hypocotyl growth in the dark. Previous studies have shown that Arabidopsis seedlings germinated under either light or dark conditions look similar during the first 2 d (Wei et al., 1994). Under both conditions, a burst of hypocotyl growth occurs mainly between days 3 and 5 postgermination, but this burst is much more pronounced in dark-grown seedlings (Wei et al., 1994; Gendreau et al., 1997). As a result, dark-grown seedlings exhibit elongated hypocotyls 4 d after seed germination, while hypocotyl growth in light-grown seedlings is comparatively limited (Wei et al., 1994); much of this growth inhibition in R is due to the action of phyB (Reed et al., 1994). Given these reports, an assay was designed to determine whether photobodies are involved in regulating the magnitude of this growth burst in the dark. In this assay, seedlings were first exposed to 10 µmol m−2 s−1 continuous R for 48 h, which allows for seed germination and for phyB to localize to large photobodies (Chen et al., 2003). Then, the seedlings were transferred to the dark just before the presumed growth burst for an additional 144 h (6 d), during which time the seedling growth kinetics were monitored by measuring hypocotyl length at various time points (Fig. 1A). We reasoned that if photobodies play a role in regulating hypocotyl growth in the dark, then the PBG and NGB lines should exhibit different hypocotyl growth kinetics in this assay. Consistent with previous reports (Wei et al., 1994; Gendreau et al., 1997), hypocotyl growth in both the PBG and NGB lines occurred mainly during the first 2 d after the R-to-D transition (equivalent to days 3 and 4 after stratification; Fig. 1, B and C). Interestingly, PBG seedlings were able to repress hypocotyl growth more efficiently and thus were much shorter than NGB seedlings in this condition (Fig. 1, B and C).
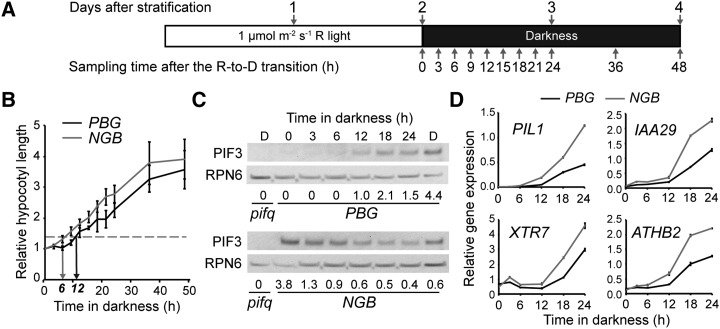
Figure 1.
PBG represses hypocotyl growth substantially longer than NGB after the R-to-D transition. A, Schematic of the R-to-D transition experiment. Seedlings were collected at the indicated time points after the R-to-D transition, and hypocotyl lengths were measured. B, Absolute hypocotyl lengths of PBG (black bars) and NGB (gray bars) at the time points shown in A. Error bars indicate the sd of at least 30 seedlings. C, Hypocotyl lengths of PBG (black line) and NGB (gray line) relative to those at time zero. D, Schematic of the experimental conditions for assessing the fine-scale growth kinetics of PBG and NGB. E, Growth kinetics of PBG (black line) and NGB (gray line) seedlings grown in the conditions shown in D. The horizontal dotted line indicates a relative hypocotyl length of 1.3, the threshold for considering a seedling as having grown. The black and gray arrows indicate the time points at which PBG and NGB cross this threshold, 18 and 6 h, respectively, after the R-to-D transition. Error bars represent the se of three independent experiments. F, Growth kinetics of Col-0, phyB-9, and pifq seedlings grown in the conditions shown in D. The horizontal dotted line indicates a relative hypocotyl length of 1.3. The arrow indicates the time point at which Col-0 crosses this threshold, 6 h, after the R-to-D transition. Error bars represent the sd of at least 15 seedlings.
Because our initial experiments showed that hypocotyl growth occurs mainly during the first 2 d after the R-to-D transition, we refined the assay and performed more detailed hypocotyl growth kinetics analysis on PBG and NGB during the first 48 h after the R-to-D transition (Fig. 1, D and E). We defined the capacity to repress hypocotyl growth as the period of time that a seedling could maintain its hypocotyl length to within an arbitrary threshold of 1.3-fold that of time zero. These experiments showed that the PBG line was able to repress hypocotyl growth for 18 h in the dark. In contrast, the NGB line was only able to repress hypocotyl growth for 6 h (Fig. 1E). Therefore, under this experimental condition, the PBG line could repress hypocotyl growth three times longer than the NGB line. This result was surprising because, in terms of hypocotyl growth inhibition, the NGB line is hypersensitive to light (Matsushita et al., 2003). Because the protein levels of PBG and NGB remained relatively constant during the course of the assay (Supplemental Fig. S1), and because a major difference between PBG and NGB is that PBG, but not NGB, can localize to photobodies, these results suggest that photobody localization of PBG might be the cause of the difference in the capacity for hypocotyl growth inhibition between the PBG and NGB lines.
Because both PBG and NGB are transgenic lines overexpressing either PBG or NGB, we wanted to confirm that hypocotyl growth in the R-to-D transition assay is also repressed by phyB and promoted by PIFs in the wild type. Therefore, we examined the growth kinetics of the wild-type Columbia-0 (Col-0), phyB-9, and pifq in our assays (Fig. 1F). Col-0 seedlings were able to repress hypocotyl growth for 6 h, and this hypocotyl growth repression was almost completely lost in phyB-9 (Fig. 1F), suggesting that hypocotyl growth repression during the R-to-D transition is phyB dependent. The fact that PBG can repress hypocotyl growth longer than the wild-type Col-0 (Fig. 1, E and F) suggests that the amount of phyB is important in determining the capacity for hypocotyl growth inhibition in the dark. In contrast to Col-0 and phyB-9, pifq was impaired in growth after the R-to-D transition (Fig. 1F), indicating that the hypocotyl growth after the R-to-D transition is mediated by PIFs.
PBG, But Not NGB, Can Repress PIF3 Accumulation in Both the Light and the Dark
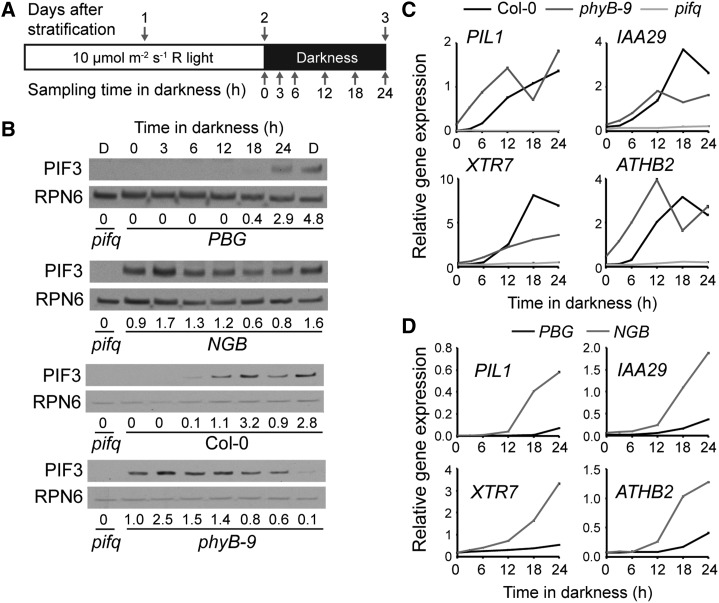
Among the PIFs, PIF3 plays an import role in promoting hypocotyl growth in the dark under diurnal conditions; in particular, PIF3 is not regulated at the transcriptional level by the circadian clock but mainly at the step of protein degradation by photoactivated phytochromes (Soy et al., 2012). Therefore, we decided to use PIF3 as a model to examine whether phytochrome-mediated PIF3 degradation is differentially regulated in the PBG and NGB lines during the R-to-D transition. In the PBG line, PIF3 was undetectable in the light and remained that way until 18 h after the R-to-D transition (Fig. 2, A and B). Consistent with the model that PIF3 promotes hypocotyl growth, the appearance of PIF3 was perfectly correlated with the increase in hypocotyl growth in both PBG and Col-0 (Fig. 2B). In striking contrast, PIF3 accumulated in NGB and phyB-9 in continuous R and after the R-to-D transition (Fig. 2B); our observation that the NGB line fails to degrade PIF3 in the light is consistent with a recent report from Park et al. (2012). Because the steady-state mRNA levels of PIF3 were comparable between PBG and NGB seedlings during the R-to-D transition (Supplemental Fig. S2), the difference in PIF3 abundance between these two lines is most likely due to differences in PIF3 degradation by PBG and NGB. Together, these results show that PBG, but not NGB, can repress PIF3 accumulation both in R and in darkness.
Figure 2.
PBG, but not NGB, can repress PIF3 in the light and the dark. A, Schematic of the growth conditions and collection time points for the assay. B, PIF3 abundance in PBG, NGB, Col-0, and phyB-9. PIF3 abundance relative to the mean overall PIF3 level within each line is shown below the blots. RPN6 was used as a loading control. Lane D shows a dark-grown control. C, Expression of four well-defined PIF target genes in Col-0, phyB-9, and pifq. Data were normalized to the expression of PP2A. D, Expression of four well-defined PIF target genes in PBG and NGB. Data were normalized to the expression of PP2A. Error bars in C and D indicate the sd of three replicates.
Next, to assess the transcriptional activity of PIF3 in the PBG and NGB lines at various time points during the R-to-D transition, we determined the expression levels of four well-characterized PIF target genes: PHYTOCHROME-INTERACTING FACTOR3-Like1 (PIL1), INDOLE-3-ACETIC ACID INDUCIBLE29 (IAA29), XYLOGLUCAN EDOTRANSGLYCOSYLASE7 (XTR7), and ARABIDOPSIS THALIANA HOMEOBOX PROTEIN2 (ATHB2) (Leivar et al., 2009, 2012b; Hornitschek et al., 2012). None of these genes were activated in pifq (Fig. 2C), confirming that, in this assay condition, their expression is dependent on PIFs. The expression of the PIF3 target genes was also correlated with the PIF3 levels in Col-0 and phyB-9, as all four genes were induced between 6 and 12 h after the R-to-D transition in Col-0, and they were induced in phyB-9 compared with Col-0 at time zero (Fig. 2C). In PBG, the four PIF target genes were induced between 18 and 24 h after the R-to-D transition (Fig. 2, B and D). Therefore, the timing of this induction corresponded faithfully to the increase in PIF3 levels and the initiation of hypocotyl growth (Figs. 1, E and F, and 2, B and D). In contrast, the induction of PIF targets did not coincide with PIF3 protein accumulation in the NGB line; although PIF3 was present at all time points, the PIF targets were only induced between 6 and 12 h after the R-to-D transition (Fig. 2, B and D). Therefore, in NGB, it is not the PIF protein level, but rather the activity of PIF3, that correlates with the initiation of hypocotyl growth (Figs. 1E and 2, B and D). These results support the notion that there are at least two mechanisms by which phyB represses hypocotyl elongation in the dark: repression of PIF3 accumulation and inhibition of PIF3 transcriptional activity. Interestingly, PBG seems to inhibit hypocotyl growth mainly by repressing PIF3 accumulation or by regulating both PIF3 abundance and transcriptional activity simultaneously, whereas NGB inhibits hypocotyl growth primarily by inhibiting PIF transcriptional activity.
Photobody Disassembly in PBG Correlates with PIF3 Accumulation and Hypocotyl Growth
The discrepancies between PBG and NGB in hypocotyl growth kinetics, PIF3 accumulation, and the expression of PIF target genes in the R-to-D transition assay provided an opportunity to precisely determine the roles of photobodies in these processes in the dark. Previous studies have utilized two main parameters to describe the dynamics of photobodies: the percentage of nuclei with or without photobodies and the average size/number of photobodies per nucleus (Yamaguchi et al., 1999; Kircher et al., 2002; Chen et al., 2003, 2010). In the past, the size and number of photobodies have been measured primarily by using two-dimensional maximum projection images derived from stacks of images of optical sections. Although this approach is useful for the analysis of nuclei with only a few large photobodies, it does not work well for nuclei with many small photobodies; because small photobodies from different optical sections might overlap in the projected image, the information on the size and number of photobodies could be lost or misrepresented in the projection.
To circumvent this problem, we analyzed photobodies from three-dimensional stacks of confocal images using the object analysis tool of Huygens Essential software (Scientific Volume Imaging). Using the software, we determined the number of large and small photobodies per nucleus and the size distribution of the small photobodies. All objects smaller than 0.5E-3 μm3 in volume (0.1 μm in estimated diameter, assuming that photobodies are spherical) were excluded from our measurements because these objects were beyond our detection limit. We arbitrarily defined large photobodies as those with a volume equal to or greater than 0.2 μm3 (0.72 μm in estimated diameter) and small photobodies as those with a volume between 0.5E-3 and 0.2 μm3 (0.1–0.72 μm in estimated diameter).
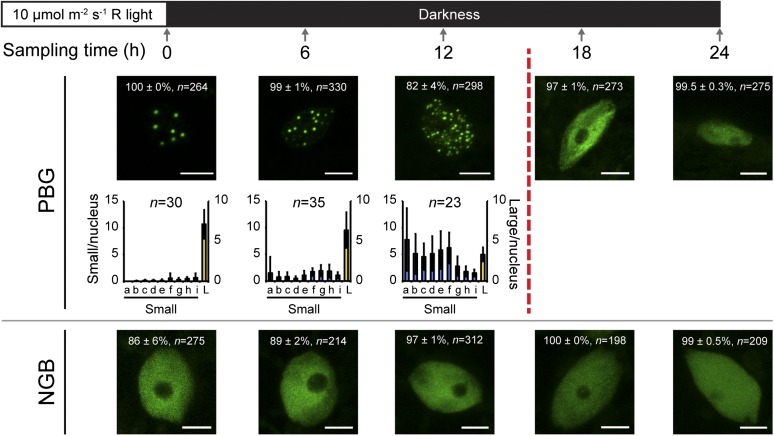
Because the major differences between PBG and NGB in hypocotyl growth repression occur during the first 24 h after the R-to-D transition (Fig. 1E), we focused on the dynamics of PBG and NGB localization during this time period. As expected, PBG seedlings grown in continuous R for 2 d had photobodies in all hypocotyl nuclei; on average, there were between six and eight large photobodies per nucleus (Fig. 3). Some nuclei also had a few small photobodies, but these nuclei were rare (Fig. 3). After the R-to-D transition, the photobody morphology in PBG went through two major transitions. The first transition took place over the first 12 h in darkness; during this period, although most of the hypocotyl nuclei had photobodies, the large photobodies disassembled and began to disappear and the number of small photobodies increased (Fig. 3). The second transition occurred between 12 and 18 h after the R-to-D transition; during this period, photobodies were completely lost from about 97% of hypocotyl nuclei (Fig. 3). The disappearance of photobodies at 18 h coincides with the accumulation of PIF3 (Fig. 2B) and the initiation of hypocotyl growth in PBG (Fig. 1E). Therefore, these data support the model that photobodies are required for inhibiting hypocotyl growth by repressing PIF3 in the dark. The analysis of the NGB line further supports this notion. As shown in Figure 3, the majority of nuclei in NGB did not contain any photobodies. Inconsistent with the report by Matsushita et al. (2003), a small fraction of nuclei did contain small photobodies under these experimental conditions. Nonetheless, because NGB failed to localize to photobodies in more than 80% of nuclei (Fig. 3), these results are consistent with the idea that photobodies are required for PIF degradation in the light and the prolonged repression of PIF accumulation in the dark. Our data also suggest that the repression of PIF3 activity by NGB can occur in the absence of photobodies.
Figure 3.
Loss of photobodies correlates with the accumulation of PIF3. Top, schematic of experimental conditions and sampling time points. Bottom, representative confocal images of PBG (top row) and NGB (bottom row) localization, along with the quantification of photobody number and size in conditions in which at least 50% of nuclei have photobodies. After 12 h in darkness, PBG begins to accumulate PIF3 and PIF target genes are induced (broken vertical red line; Fig. 2). In the confocal images, the percentage value indicates the mean percentage of all analyzed nuclei with the phenotype shown in the image (with or without photobodies; means ± se of at least three independent experiments); n indicates the total number of nuclei analyzed to generate the percentage, and bars = 5 μm. In the graphs, the error bars represent sd and n indicates the number of nuclei analyzed to generate the distribution. The blue bars indicate small photobodies (diameters between 0.1 and 0.72 µm; volumes between 0.0005 and 0.19 µm3), and the yellow bars, plotted on the secondary axis, indicate large photobodies (diameters greater than 0.72 µm; volumes of 0.2 µm3 or greater). Bins represent the following volume ranges, with a to i representing small photobodies and L representing large photobodies: a, 0.0005 to 0.0019; b, 0.002 to 0.0049; c, 0.005 to 0.099; d, 0.01 to 0.019; e, 0.02 to 0.039; f, 0.04 to 0.079; g, 0.08 to 0.119; h, 0.12 to 0.159; i, 0.16 to 0.199; and L, 0.20 μm3 or greater.
Decreased Light Intensity Leads to the Faster Disassembly of Photobodies and a Reduced Capacity for Hypocotyl Growth Inhibition in PBG in the Dark
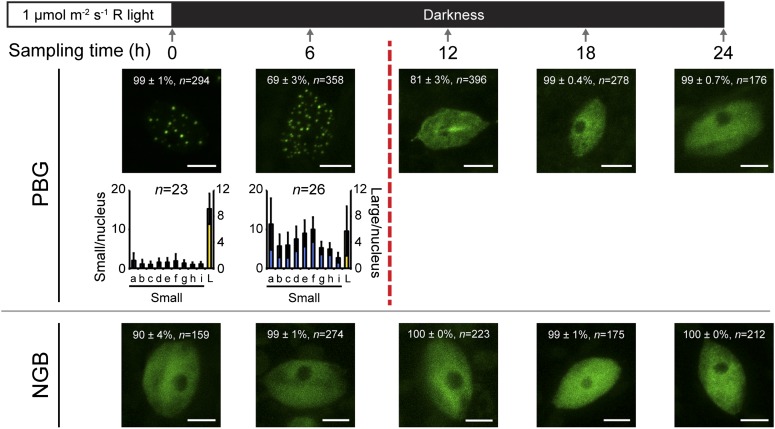
To further test the model that photobody morphology determines the capacity for PIF3 repression and hypocotyl inhibition in the dark, we asked whether we could alter these two latter processes by manipulating photobody morphology. Because the steady-state pattern of photobodies is directly regulated by light intensity (Chen et al., 2003; Van Buskirk et al., 2012), we modified our assay condition by growing seedlings in a reduced R intensity of 1 µmol m−2 s−1 for 2 d before the R-to-D transition. As reported previously (Chen et al., 2003), in the dimmer light condition, PBG was localized to both large and small photobodies (Fig. 4). Compared with the 10 µmol m−2 s−1 R treatment, the dimmer light treatment led to the faster disassembly of PBG photobodies in the dark; this difference was most obvious between the 6- and 12-h time points (Fig. 4). At the 6-h time point, the percentage of nuclei with photobodies had already dropped to approximately 69%; at the 12-h time point, photobodies were completely lost from more than 80% of all nuclei. Therefore, the photobody disassembly process in PBG was at least 6 h faster after the 1 µmol m−2 s−1 R treatment compared with the 10 µmol m−2 s−1 R treatment. In contrast, the localization pattern of NGB was quite similar after both the strong and dim R treatments (Figs. 3 and 4).
Figure 4.
Seedlings grown in a lower fluence rate of light lose photobodies more quickly than in a higher fluence rate of light. Top, schematic of experimental conditions and sampling time points. Bottom, representative confocal images of PBG (top row) and NGB (bottom row) localization, along with the quantification of photobody number and size in conditions in which at least 50% of nuclei have photobodies. After 6 h in darkness, PBG loses photobodies from more than 50% of nuclei (broken vertical red line). In the confocal images, the percentage value indicates the mean percentage of all analyzed nuclei with the phenotype shown in the image (with or without photobodies; means ± se of at least three independent experiments); n indicates the number of nuclei analyzed to generate the percentage, and bars = 5 μm. In the graphs, the error bars represent sd and n indicates the number of nuclei analyzed to generate the distribution. The blue bars indicate small photobodies (diameters between 0.1 and 0.72 µm; volumes between 0.0005 and 0.19 µm3), and the yellow bars, plotted on the secondary axis, indicate large photobodies (diameters greater than 0.72 µm; volumes of 0.2 µm3 or greater). Bins represent the following volume ranges, with a to i representing small photobodies and L representing large photobodies: a, 0.0005 to 0.0019; b, 0.002 to 0.0049; c, 0.005 to 0.099; d, 0.01 to 0.019; e, 0.02 to 0.039; f, 0.04 to 0.079; g, 0.08 to 0.119; h, 0.12 to 0.159; i, 0.16 to 0.199; and L, 0.20 µm3 or greater.
To test whether the change in photobody dynamics in PBG leads to changes in the kinetics of PIF3 accumulation, PIF transcriptional activity, and hypocotyl growth, we examined these responses in both PBG and NGB during the 1 µmol m−2 s−1 R-to-D transition (Fig. 5A). As shown in Figure 5, consistent with the loss of photobodies in PBG after 12 h in darkness, the PBG seedlings grown in 1 µmol m−2 s−1 R were only able to repress hypocotyl growth, PIF3 accumulation, and the expression of PIF targets for approximately 10 to 12 h (Fig. 5, B–D). In contrast, just as NGB exhibited similar localization patterns between the 1 and 10 µmol m−2 s−1 treatments (Figs. 3 and 4), NGB seedlings showed similar hypocotyl growth kinetics, PIF3 accumulation, and PIF target gene induction between the two light conditions (Figs. 1E, 2, B and D, and 5, B–D). Together, these data suggest that the steady-state pattern of photobodies in PBG prior to the R-to-D transition correlates with the capacity to fine-tune hypocotyl growth inhibition and PIF3 repression in the dark. Consistent with this notion, NGB, which does not localize to photobodies in the majority of nuclei, does not respond to differences in light quantity before the dark period.
Figure 5.
PBG has a reduced capacity for hypocotyl growth inhibition and PIF3 repression after a dimmer, 1 μmol m−2 s−1 R-to-D transition. A, Schematic of the growth conditions and sampling time points for the assay. B, Growth kinetics of PBG (black line) and NGB (gray line). The horizontal dotted line indicates the threshold value of 1.3, and black and gray arrows point to where PBG and NGB cross that threshold (at 12 and 6 h, respectively). Error bars represent the se of three independent experiments. C, Western blots showing PIF3 abundance in PBG (top) and NGB (bottom). PIF3 levels relative to the mean overall PIF3 level within each line are shown below the blots. RPN6 was used as a loading control. Lane D shows a dark-grown control. D, Transcript levels of four well-defined PIF target genes in PBG (black lines) and NGB (gray lines). Data were normalized to PP2A. Error bars represent the sd of three replicates.
The Prolonged Hypocotyl Growth Inhibition in PBG Is Likely Due to Enhanced Stabilization of the Pfr of phyB
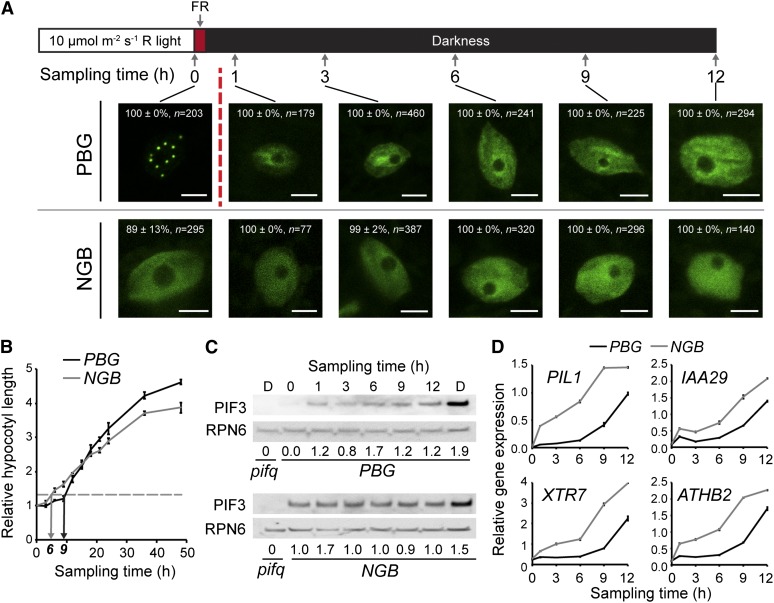
Why, compared with NGB, can PBG repress PIF3 accumulation and hypocotyl growth for a prolonged period of time in the dark? One possible explanation could come from differences in the stability of the Pfr of phyB. Although the dark reversion rate of NGB is similar to that of full-length phyB in vitro (Oka et al., 2004), the dark reversion rate of full-length phyB in vivo is much slower; it has been proposed that, in vivo, photobodies can stabilize the Pfr form of phyB (Rausenberger et al., 2010). To test this hypothesis, we treated PBG and NGB seedlings with a 15-min FR pulse to convert PBG and NGB to their respective Pr before transferring them to darkness. Because photobody localization of phyB is Pfr dependent, FR treatment should trigger the fast disassembly of photobodies in the dark (Rausenberger et al., 2010; Ádám et al., 2011). To monitor this rapid change in photobody disassembly, we examined photobody dynamics at time points immediately after the FR treatment (Fig. 6A). Consistent with previous reports (Rausenberger et al., 2010; Ádám et al., 2011), almost all photobodies in PBG disassembled within 1 h of the FR treatment (Fig. 6B). The small fraction of cells with some small photobodies in NGB also lost their photobodies within 1 h, indicating that the small photobodies in NGB are also dependent on its Pfr.
Figure 6.
Photobody localization of PBG correlates with the repression of PIF3 accumulation, but not the repression of PIF3 activity, after the R-FR-D transition. A, Top, schematic of the experimental growth conditions and sampling time points. Bottom, representative confocal images of PBG (top row) and NGB (bottom row) localization. One hour after the FR treatment, all photobodies are gone from PBG (broken vertical red line). The percentage values indicate the percentage of all analyzed nuclei with the phenotype shown in the image (with or without photobodies; means ± sd of at least two independent experiments); n indicates the number of nuclei analyzed to generate the percentage, and bars = 5 µm. B, Growth kinetics of PBG (black line) and NGB (gray line). The horizontal dotted line is the threshold value of 1.3, and black and gray arrows point to where PBG and NGB cross this threshold after FR treatment: 9 and 6 h, respectively. Error bars indicate the se of three independent experiments. C, Western blot showing PIF3 abundance in PBG (top) and NGB (bottom) after FR treatment. PIF3 levels relative to the mean overall PIF3 level within each line are given below the blots. RPN6 was used as a loading control. Lane D shows a dark-grown control. D, Transcript levels of four well-defined PIF target genes in PBG (black lines) and NGB (gray lines) after FR treatment. Data were normalized to PP2A. Error bars represent the sd of three replicates.
Measuring hypocotyl growth kinetics after FR treatment showed that the FR pulse treatment caused virtually no change in the growth kinetics of NGB, which still began to grow 6 h after the red light-to-far-red light-to-dark (R-FR-D) transition (Fig. 6B), suggesting that the minimum time required for a 30% increase in hypocotyl length might be approximately 6 h. In contrast, PBG responded strongly to the FR treatment; hypocotyl growth was inhibited for only approximately 9 h, which is half the time of that without FR treatment (Figs. 1E and 6B). This result suggests that the prolonged hypocotyl growth repression in PBG (18 h, compared with 6 h in NGB) is mainly due to Pfr stabilization in the dark. However, even after the FR treatment, PBG was still able to repress hypocotyl growth 3 h longer than NGB (Fig. 6B), suggesting that there must be other mechanisms that account for this difference in hypocotyl growth repression.
We next determined the patterns of PIF3 accumulation and the expression of PIF targets in both PBG and NGB after the R-FR-D transition. In PBG, PIF3 began to accumulate within 1 h after the FR treatment (Fig. 6C); this result is consistent with a previous report on PIF3 dynamics (Monte et al., 2004). The accumulation of PIF3 in the PBG line again correlated perfectly with photobody disassembly (Fig. 6, A and C). Because PIF3 degradation is triggered by the Pfr of phyB, the dynamic changes in PIF3 levels could serve as a readout for the presence of the Pfr of phyB in PBG. Based on this readout, the Pfr of phyB-GFP in PBG lasts for approximately 18 h after the 10 µmol m−2 s−1 R-to-D transition (Fig. 2B) and for about 12 h after the 1 µmol m−2 s−1 R-to-D transition (Fig. 5C). As predicted, in NGB, PIF3 was detectable in continuous R and remained detectable for the duration of the experiment, showing little change in abundance after FR treatment (Fig. 6C).
Surprisingly, although PIF3 began to accumulate in PBG within 1 h of the FR treatment, the expression of PIF targets remained repressed for 9 h (Fig. 6D). In contrast, in NGB, the expression of all four PIF targets was induced immediately after FR treatment (Fig. 6E). These data suggest that the repression of PIF activity in NGB in the dark is mainly dependent on the Pfr of NGB; however, in PBG, the repression of the expression of PIF targets could be mediated by an unknown mechanism that is independent of photobodies and of the Pfr of phyB. This offers an explanation for the difference in hypocotyl growth inhibition between the PBG and NGB lines during the R-FR-D transition (Fig. 6B). Because the expression of PIF target genes is Pfr dependent in NGB, the repression of PIF target genes can be used as a readout for the presence of the Pfr of NGB; based on this readout, the Pfr of NGB can last for approximately 6 h after both the 10 and 1 µmol m−2 s−1 R-to-D transitions (Figs. 2D and 5D). Together, these results suggest that the Pfr of PBG can last 12 h longer than that of NGB.
DISCUSSION AND CONCLUSION
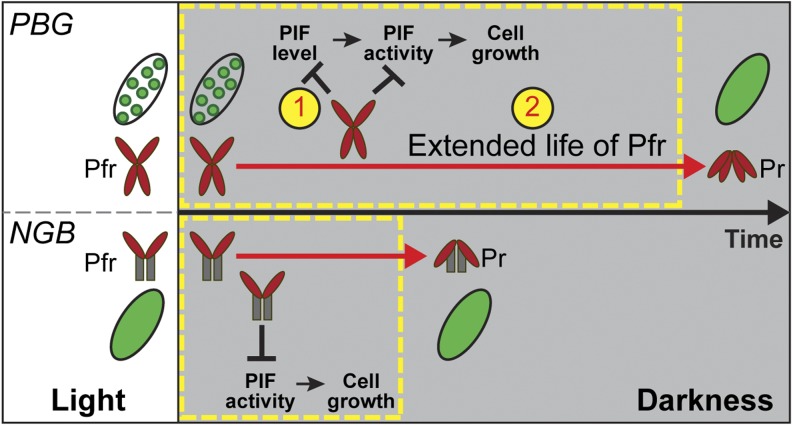
Although a growing body of evidence supports the biological importance of photobodies in the phytochrome-mediated photoinhibition of hypocotyl growth (Van Buskirk et al., 2012), comparisons between the photobody-localized PBG and nucleoplasm-localized NGB showed that, in the light, NGB is hyperactive in inhibiting hypocotyl growth, suggesting that photobodies are unnecessary and might even play a negative role in the light-dependent inhibition of hypocotyl elongation (Matsushita et al., 2003; Palágyi et al., 2010). Here, we developed an R-to-D transition assay to examine the relationship between dynamic changes in photobody morphology, the molecular events of PIF3 accumulation, and the expression of PIF targets as well as the repression of hypocotyl growth between the PBG and NGB lines. Our results demonstrate a tight correlation between the photobody localization of PBG and the repression of PIF3 accumulation in both light and dark conditions. Our data support a model in which photobodies mediate prolonged, light-dependent hypocotyl growth inhibition in the dark by stabilizing the Pfr of phyB and repressing PIF3 accumulation (Fig. 7).
Figure 7.
Model for the function of photobodies in regulating seedling growth in the dark. The Pfr of both PBG and NGB can persist into the darkness to repress hypocotyl growth. There are two main differences between the photobody-localized PBG and the nucleoplasmic NGB. (1) Photobodies are required for PIF degradation; photobody localization of PBG inhibits both PIF accumulation and PIF transcriptional activity, whereas NGB only represses PIF activity. (2) Photobody localization of PBG stabilizes its Pfr and extends its life in the dark; consequently, PBG can inhibit hypocotyl elongation (cell growth) for a prolonged period of time. This photobody-dependent mechanism of Pfr stabilization enables seedlings to convey perceived light cues into darkness to fine-tune hypocotyl growth accordingly.
Photobody Localization of phyB Tightly Correlates with the Repression of PIF3 Degradation
Accumulating evidence suggests that phytochromes inhibit hypocotyl growth both by triggering the degradation of multiple PIFs and by inhibiting the PIFs’ transcriptional activity. Our data suggest that photobody localization of phyB is specifically required for repressing PIF3 accumulation in the light and after the light-to-dark transition. This conclusion is supported by the following two lines of evidence: first, by comparing PIF3 levels in 2-d-old light-grown PBG and NGB seedlings, we show that PIF3 accumulation is repressed in PBG but not in NGB (Fig. 2B). This result confirms the observations from previous reports by Choi and colleagues, who showed, using transgenic lines overexpressing His- and Myc-tagged PIF3, that NGB fails to degrade PIF3 in continuous R (Park et al., 2004, 2012). Second, in all three conditions tested, the 10 µmol m−2 s−1 R-to-D transition (Figs. 2B and 3), the 1 µmol m−2 s−1 R-to-D transition (Figs. 4 and 5C), and the R-FR-D transition (Fig. 6. A and C), the disassembly of photobodies in hypocotyl nuclei correlates tightly with the accumulation of PIF3. The R-FR-D experiment is particularly informative, as this treatment demonstrates that the regulation of PIF degradation and the repression of PIF activity can be separated even in the PBG background.
The tight correlation between the photobody localization of PBG and PIF3 degradation provides strong evidence supporting the model that photobody localization of phyB is required for PIF3 degradation (Fig. 7). Although we still cannot completely exclude the possibility that photobody localization of phyB and PIF3 degradation are two parallel consequences of phyB activation, the conclusion that photobodies are required for PIF degradation is consistent with and supported by previously published results. First, the constitutively active phyB allele, YHB, localizes to photobodies and can trigger PIF3 degradation in the dark (Su and Lagarias, 2007; Galvão et al., 2012). Second, the photobody-deficient mutant, hmr, is also defective in the degradation of PIF1 and PIF3 in the light (Chen et al., 2010). In addition, in the YHB/hmr-1 double mutant, YHB fails to localize to large photobodies in the dark and the repression of PIF3 accumulation by YHB is also reversed (Galvão et al., 2012). Third, PIF3 localizes to photobodies prior to its degradation (Bauer et al., 2004; Al-Sady et al., 2006), and mutations in phyB that abrogate its interaction with PIFs do not affect phyB’s localization to photobodies but are not functional in the photoinhibition of hypocotyl elongation (Oka et al., 2008; Kikis et al., 2009), suggesting that recruiting PIFs to photobodies is required for PIF degradation and phytochrome signaling. Taken together, the results from this and previous studies support the most likely model that photobodies are required for PIF degradation.
The molecular mechanism by which photobodies are involved in PIF degradation is still unclear. There is still no direct evidence showing that PIF3 degradation occurs on photobodies. Therefore, we cannot exclude the possibility that photobodies are only involved in a posttranslational modification of PIFs, such as phosphorylation (Al-Sady et al., 2006; Lorrain et al., 2008; Shen et al., 2008; Bu et al., 2011; Ni et al., 2013), and that PIF degradation occurs elsewhere (Van Buskirk et al., 2012). It is also important to note that although our data show a clear separation between PIF3 degradation and the regulation of its transcriptional activity in the R-FR-D transition (Fig. 6, C and D), these two events occurred simultaneously in the other two R-to-D conditions (Figs. 2, B and D, and 5, C and D). Therefore, it is possible that PIF degradation and the regulation of PIF target genes are closely linked in the presence of photobodies (Fig. 7).
It is worth pointing out that the repression of PIF3 activity after the R-FR-D transition in PBG appears to be mediated by a different mechanism from the NGB-dependent repression of PIF3 activity. It has previously been shown that NGB inhibits PIF3 activity by removing PIF3 from the promoters of its target genes and that this activity of NGB is Pfr dependent (Park et al., 2012). Our results are consistent with this report in that an FR pulse can release the repression of PIF targets by NGB (Fig. 6D). In contrast, the repression of PIF targets in PBG after the R-FR-D transition was clearly not mediated by the Pfr of PBG, as the FR pulse quickly converted PBG to its Pr and promoted PIF accumulation, but the repression of PIF targets persisted (Fig. 6D). Therefore, the repression of PIF activity in PBG in the assay is mediated by a yet unknown mechanism. One possibility is that another transcriptional repressor protein for the PIF targets is present only in PBG and the delay in the induction of the PIF targets reflects the time required for the removal of this additional protein. An alternative model is that the chromatin state of the PIF targets is different between the PBG and NGB lines in the light; while the PIF targets could be primed for induction in NGB, in PBG they might be in a silenced state that requires additional time for activation. Future experiments on the chromatin status of the PIF targets in these two lines might help to reveal this unknown mechanism for the repression of PIF target genes after the R-FR-D transition.
Photobodies Mediate Prolonged Hypocotyl Growth Inhibition in the Dark Likely by Stabilizing the Pfr of phyB
It is well known that the Pfr of phyB has a relatively slow dark reversion rate and that it can persist into darkness; these properties of phyB play a pivotal role in hypocotyl growth inhibition under diurnal conditions (Hennig et al., 1999; Rausenberger et al., 2010). This is best demonstrated by end-of-day FR treatment, in which an FR light pulse is applied at dusk to inactivate phyB to its Pr. End-of-day FR treatment leads to a dramatic decrease in hypocotyl growth inhibition under diurnal conditions (Elich and Chory, 1997). Recently, it has been shown that the Pfr of phyB is responsible for repressing PIF1 and PIF3 accumulation and, consequently, hypocotyl growth in the dark (Soy et al., 2012, 2014). Interestingly, the in vivo dark reversion rate of phyB is much slower than the rate measured in vitro, and photobodies have been proposed through mathematical modeling to be involved in stabilizing the Pfr of phyB in the dark (Rausenberger et al., 2010). Here, we provide experimental evidence supporting this hypothesis. Our data show that the localization of PBG to photobodies dramatically increases the stability of its Pfr compared with that of the nucleoplasmic NGB. First, because the function of the Pfr of PBG is closely associated with the repression of PIF3 accumulation, we can estimate the presence of PBG Pfr based on PIF3 levels. Similarly, because the function of the Pfr of NGB is closely linked to the repression of PIF target gene expression, we can estimate the presence of NGB Pfr based on the induction of PIF targets. Based on the comparison between the 10 µmol m−2 s−1 R-to-D transition experiment (Fig. 2, B and D) and the R-FR-D transition experiment (Fig. 6, C and D), the Pfr of PBG lasts for about 18 h compared with only 6 h for the Pfr of NGB. Because PBG and NGB have similar dark reversion rates in vitro (Oka et al., 2004), our data suggest that the life of the Pfr of PBG is extended three times longer than that of NGB in vivo. This dramatic decrease in the capacity for hypocotyl growth inhibition in PBG between the 10 µmol m−2 s−1 R-to-D transition (Fig. 1E) and the R-FR-D transition (Fig. 6B) demonstrates that the stabilization of the Pfr of PBG contributes substantially to the prolonged inhibition of hypocotyl growth in the dark. The stability of PBG Pfr can also be fine-tuned by the light intensity and photobody localization pattern before the light-to-dark transition; in the 1 µmol m−2 s−1 R-to-D transition experiment, PBG localized to large and small photobodies and the life of the Pfr was reduced to 9 h (Figs. 4 and 5). In contrast, the life of the Pfr of NGB seems to remain the same in both the 10 and 1 µmol m−2 s−1 R-to-D transitions (Figs. 2D and 5D). Together, these results suggest that the photobody localization of phyB is required for the prolonged inhibition of hypocotyl growth in the dark by stabilizing the Pfr of phyB and that this photobody-dependent Pfr stabilization could be a mechanism to fine-tune hypocotyl elongation in the dark (Fig. 7).
Although our experiments were not performed under diurnal conditions, the mechanism involved in hypocotyl growth regulation appears to be similar between the R-to-D transition and the day-to-night transition; under both conditions, hypocotyl growth is promoted by PIFs and repressed by active phyB (Figs. 1F and 2, B and C; Soy et al., 2012, 2014). Because photobodies also undergo similar disassembly dynamics during the day-to-night transition (Kircher et al., 2002), in diurnal conditions the photobody localization of phyB may be involved in the PIF repression and hypocotyl growth inhibition seen in the early evening. We propose that this photobody-dependent hypocotyl growth repression mechanism allows seedlings to carry light cues perceived during the day into the evening to fine-tune photomorphogenetic responses accordingly (Fig. 7).
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Hypocotyl Measurement
The PBG (Yamaguchi et al., 1999), NGB (Matsushita et al., 2003), phyB-9 (Reed et al., 1993), and pifq (Leivar et al., 2008) lines of Arabidopsis (Arabidopsis thaliana) were as described previously. Seeds were surface sterilized with a rinse in 70% (v/v) ethanol followed by 15 min in 50% (v/v) bleach supplemented with 0.02% (v/v) Triton X-100. Seeds were rinsed five times with distilled, deionized water prior to plating on one-half-strength Murashige and Skoog medium supplemented with B vitamins (Caisson) and containing 0.6% (w/v) phyto agar (Caisson). Seeds were stratified for 5 d in darkness prior to being treated with the indicated light conditions. The light intensity in the R and FR light-emitting diode chambers (Percival Scientific) was measured using a fiber-optic probe and SpectraWiz software (StellarNet). Seedling images were obtained by laying seedlings on a transparency and scanning using the Epson Perfection V700 photo scanner. Hypocotyl lengths were measured using ImageJ software (http://rsbweb.nih.gov/ij/).
Protein Extraction and Western Blot
Total protein was extracted from seedlings using a mortar and pestle and 3 volumes of extraction buffer containing Bromphenol Blue as described previously (Galvão et al., 2012). Protein samples were run on 8% (w/v) Bis-Tris-SDS-acrylamide gels and transferred to nitrocellulose membranes (Bio-Rad). Polyclonal antibodies against PIF3 (Chen et al., 2010) were used at a 1:500 dilution, and polyclonal antibodies against REGULATORY PARTICLE NON-ATPASE6 (Enzo Life Sciences; catalog no. BML-PW8370-0025) were used at a 1:1,000 dilution. Secondary antibodies were horseradish peroxidase-conjugated goat anti-rabbit IgG (Bio-Rad) and were used at a 1:5,000 dilution. Blots were visualized on x-ray film using SuperSignal West chemiluminescent substrate (Thermo Fisher Scientific). PIF3 and GFP levels were quantified using QuantityOne software (Bio-Rad) followed by a multistep normalization: after background subtraction, the intensity of the PIF3 or GFP band was divided by the intensity of the corresponding REGULATORY PARTICLE NON-ATPASE6 band. Then, the mean PIF3 or GFP intensity was calculated, and the normalized PIF3 or GFP intensity for each individual time point within each line was divided by this mean. This is the value that is reported in the figures.
Quantitative Reverse Transcription-PCR
Seedlings were flash frozen in liquid nitrogen and ground to a powder using a plastic mortar and wooden pestle. Total RNA was then extracted using the Spectrum Plant Total RNA kit (Sigma-Aldrich; catalog no. STRN-250) with on-column DNase treatment (Sigma-Aldrich; catalog no. DNASE10-1SET), and yield was quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). Five micrograms of total RNA was then used for complementary DNA synthesis using SuperScript II Reverse Transcriptase (Life Technologies; catalog no. 18064-014) using oligo(dT)12-18 primers (Life Technologies; catalog no. 18418-012) according to the manufacturer’s instructions. Quantitative reverse transcription-PCR was performed using FastStart Universal SYBR Green (Roche Applied Science; catalog no. 04913914001) and a Mastercycler ep realplex qPCR machine with realplex software (Eppendorf). Primers are listed in Supplemental Table S1.
Confocal Live-Cell Imaging and Quantification of Photobody Morphology
Seedlings were mounted on Superfrost slides (VWR; catalog no. 48311-600) using distilled, deionized water and 22- × 40-mm coverslips (no. 1.5, VWR; catalog no. 48393-172). Nuclei from hypocotyl epidermal cells were imaged using a Zeiss LSM 510 inverted confocal microscope (Carl Zeiss). GFP was detected using a 100× Plan-Apochromat oil-immersion objective, 488-nm excitation from an argon laser, and the manufacturer’s default Green only detection setting (505- to 550-nm bandpass detector). Images were collected using LSM 510 software version 4.2. Images were processed using Adobe Photoshop CS5 software (Adobe Systems).
To calculate the overall proportion of nuclei possessing photobodies, maximum projections of optical sections of the hypocotyl cells were generated using LSM Image Browser software version 4.2.0.121 (Carl Zeiss). Then, the number of nuclei with photobodies was manually scored. To determine the size and number of photobodies, stacks of optical sections were loaded into Huygens Essential software (Scientific Volume Imaging). The object analyzer tool was used to threshold the image and to calculate the number and the volume of photobodies in the image. For each nucleus, the photobodies were sorted by size and then manually binned using Microsoft Excel.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. The protein levels of PBG and NGB remain relatively constant during the course of the R-to-D transition assay.
Supplemental Figure S2. The mRNA levels of PIF3 in PBG, NGB, Col-0, and phyB-9 lines during the R-to-D transition.
Supplemental Table S1. List of quantitative reverse transcription-PCR primers used in this study.
Supplementary Material
Acknowledgments
We thank Dr. Joanne Chory for the anti-PIF3 antibody, Dr. Peter Quail for the pifq seeds, and Drs. Sam Johnson and Yasheng Gao for technical assistance with the live-cell imaging.
Glossary
- R
red light
- FR
far-red light
- R-to-D
red light-to-dark
- Col-0
Columbia-0
- R-FR-D
red light-to-far-red light-to-dark
Footnotes
This work was supported by the National Institutes of Health (grant no. R01GM087388 to M.C.), the National Science Foundation (grant no. IOS–1051602 to M.C.), and a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology, Japan (grant no. 22120002 to A.N.).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Ádám E, Hussong A, Bindics J, Wüst F, Viczián A, Essing M, Medzihradszky M, Kircher S, Schäfer E, Nagy F. (2011) Altered dark- and photoconversion of phytochrome B mediate extreme light sensitivity and loss of photoreversibility of the phyB-401 mutant. PLoS ONE 6: e27250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sady B, Kikis EA, Monte E, Quail PH. (2008) Mechanistic duality of transcription factor function in phytochrome signaling. Proc Natl Acad Sci USA 105: 2232–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sady B, Ni W, Kircher S, Schäfer E, Quail PH. (2006) Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell 23: 439–446 [DOI] [PubMed] [Google Scholar]
- Bauer D, Viczián A, Kircher S, Nobis T, Nitschke R, Kunkel T, Panigrahi KC, Adám E, Fejes E, Schäfer E, et al. (2004) Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16: 1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Q, Zhu L, Dennis MD, Yu L, Lu SX, Person MD, Tobin EM, Browning KS, Huq E. (2011) Phosphorylation by CK2 enhances the rapid light-induced degradation of phytochrome interacting factor 1 in Arabidopsis. J Biol Chem 286: 12066–12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chory J. (2011) Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol 21: 664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chory J, Fankhauser C. (2004) Light signal transduction in higher plants. Annu Rev Genet 38: 87–117 [DOI] [PubMed] [Google Scholar]
- Chen M, Galvão RM, Li M, Burger B, Bugea J, Bolado J, Chory J. (2010) Arabidopsis HEMERA/pTAC12 initiates photomorphogenesis by phytochromes. Cell 141: 1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Schwab R, Chory J. (2003) Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc Natl Acad Sci USA 100: 14493–14498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Tao Y, Lim J, Shaw A, Chory J. (2005) Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr Biol 15: 637–642 [DOI] [PubMed] [Google Scholar]
- Elich TD, Chory J. (1997) Biochemical characterization of Arabidopsis wild-type and mutant phytochrome B holoproteins. Plant Cell 9: 2271–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Quail PH. (2010) Phytochrome functions in Arabidopsis development. J Exp Bot 61: 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori T, Yamashino T, Kato T, Mizuno T. (2004) Circadian-controlled basic/helix-loop-helix factor, PIL6, implicated in light-signal transduction in Arabidopsis thaliana. Plant Cell Physiol 45: 1078–1086 [DOI] [PubMed] [Google Scholar]
- Furuya M, Song PS (1994) Assembly and properties of holophytochrome. In RE Kendrick, GHM Kronenberg, eds, Photomorphogenesis in Higher Plants, Ed 2. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 105–140 [Google Scholar]
- Galvão RM, Li M, Kothadia SM, Haskel JD, Decker PV, Van Buskirk EK, Chen M. (2012) Photoactivated phytochromes interact with HEMERA and promote its accumulation to establish photomorphogenesis in Arabidopsis. Genes Dev 26: 1851–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H. (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig L, Poppe C, Unger S, Schäfer E. (1999) Control of hypocotyl elongation in Arabidopsis thaliana by photoreceptor interaction. Planta 208: 257–263 [DOI] [PubMed] [Google Scholar]
- Hornitschek P, Kohnen MV, Lorrain S, Rougemont J, Ljung K, López-Vidriero I, Franco-Zorrilla JM, Solano R, Trevisan M, Pradervand S, et al. (2012) Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J 71: 699–711 [DOI] [PubMed] [Google Scholar]
- Huq E, Al-Sady B, Hudson M, Kim C, Apel K, Quail PH. (2004) Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305: 1937–1941 [DOI] [PubMed] [Google Scholar]
- Huq E, Quail PH. (2002) PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J 21: 2441–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami C, Lorrain S, Hornitschek P, Fankhauser C. (2010) Light-regulated plant growth and development. Curr Top Dev Biol 91: 29–66 [DOI] [PubMed] [Google Scholar]
- Khanna R, Huq E, Kikis EA, Al-Sady B, Lanzatella C, Quail PH. (2004) A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell 16: 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikis EA, Oka Y, Hudson ME, Nagatani A, Quail PH. (2009) Residues clustered in the light-sensing knot of phytochrome B are necessary for conformer-specific binding to signaling partner PIF3. PLoS Genet 5: e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S, Gil P, Kozma-Bognár L, Fejes E, Speth V, Husselstein-Muller T, Bauer D, Adám E, Schäfer E, Nagy F. (2002) Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell 14: 1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Monte E, Cohn MM, Quail PH. (2012a) Phytochrome signaling in green Arabidopsis seedlings: impact assessment of a mutually negative phyB-PIF feedback loop. Mol Plant 5: 734–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, Huq E, Quail PH. (2008) Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol 18: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH. (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Tepperman JM, Cohn MM, Monte E, Al-Sady B, Erickson E, Quail PH. (2012b) Dynamic antagonism between phytochromes and PIF family basic helix-loop-helix factors induces selective reciprocal responses to light and shade in a rapidly responsive transcriptional network in Arabidopsis. Plant Cell 24: 1398–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Tepperman JM, Monte E, Calderon RH, Liu TL, Quail PH. (2009) Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell 21: 3535–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ljung K, Breton G, Schmitz RJ, Pruneda-Paz J, Cowing-Zitron C, Cole BJ, Ivans LJ, Pedmale UV, Jung HS, et al. (2012) Linking photoreceptor excitation to changes in plant architecture. Genes Dev 26: 785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Lorrain S, Trevisan M, Pradervand S, Fankhauser C. (2009) Phytochrome interacting factors 4 and 5 redundantly limit seedling de-etiolation in continuous far-red light. Plant J 60: 449–461 [DOI] [PubMed] [Google Scholar]
- Matsushita T, Mochizuki N, Nagatani A. (2003) Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature 424: 571–574 [DOI] [PubMed] [Google Scholar]
- Medzihradszky M, Bindics J, Ádám E, Viczián A, Klement E, Lorrain S, Gyula P, Mérai Z, Fankhauser C, Medzihradszky KF, et al. (2013) Phosphorylation of phytochrome B inhibits light-induced signaling via accelerated dark reversion in Arabidopsis. Plant Cell 25: 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte E, Tepperman JM, Al-Sady B, Kaczorowski KA, Alonso JM, Ecker JR, Li X, Zhang Y, Quail PH. (2004) The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc Natl Acad Sci USA 101: 16091–16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A. (2010) Phytochrome: structural basis for its functions. Curr Opin Plant Biol 13: 565–570 [DOI] [PubMed] [Google Scholar]
- Nagy F, Schäfer E. (2002) Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Annu Rev Plant Biol 53: 329–355 [DOI] [PubMed] [Google Scholar]
- Ni W, Xu SL, Chalkley RJ, Pham TN, Guan S, Maltby DA, Burlingame AL, Wang ZY, Quail PH. (2013) Multisite light-induced phosphorylation of the transcription factor PIF3 is necessary for both its rapid degradation and concomitant negative feedback modulation of photoreceptor phyB levels in Arabidopsis. Plant Cell 25: 2679–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nito K, Wong CC, Yates JR, 3rd,, Chory J. (2013) Tyrosine phosphorylation regulates the activity of phytochrome photoreceptors. Cell Rep 3: 1970–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN. (2007) Rhythmic growth explained by coincidence between internal and external cues. Nature 448: 358–361 [DOI] [PubMed] [Google Scholar]
- Oh E, Kim J, Park E, Kim JI, Kang C, Choi G. (2004) PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16: 3045–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Matsushita T, Mochizuki N, Quail PH, Nagatani A. (2008) Mutant screen distinguishes between residues necessary for light-signal perception and signal transfer by phytochrome B. PLoS Genet 4: e1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Matsushita T, Mochizuki N, Suzuki T, Tokutomi S, Nagatani A. (2004) Functional analysis of a 450-amino acid N-terminal fragment of phytochrome B in Arabidopsis. Plant Cell 16: 2104–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palágyi A, Terecskei K, Adám E, Kevei E, Kircher S, Mérai Z, Schäfer E, Nagy F, Kozma-Bognár L. (2010) Functional analysis of amino-terminal domains of the photoreceptor phytochrome B. Plant Physiol 153: 1834–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Kim J, Lee Y, Shin J, Oh E, Chung WI, Liu JR, Choi G. (2004) Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol 45: 968–975 [DOI] [PubMed] [Google Scholar]
- Park E, Park J, Kim J, Nagatani A, Lagarias JC, Choi G. (2012) Phytochrome B inhibits binding of phytochrome-interacting factors to their target promoters. Plant J 72: 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausenberger J, Hussong A, Kircher S, Kirchenbauer D, Timmer J, Nagy F, Schäfer E, Fleck C. (2010) An integrative model for phytochrome B mediated photomorphogenesis: from protein dynamics to physiology. PLoS ONE 5: e10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. (1994) Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol 104: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell NC, Su YS, Lagarias JC. (2006) Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol 57: 837–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. (1989) Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev 3: 1745–1757 [DOI] [PubMed] [Google Scholar]
- Shen H, Zhu L, Castillon A, Majee M, Downie B, Huq E. (2008) Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20: 1586–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Kim K, Kang H, Zulfugarov IS, Bae G, Lee CH, Lee D, Choi G. (2009) Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc Natl Acad Sci USA 106: 7660–7665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soy J, Leivar P, González-Schain N, Sentandreu M, Prat S, Quail PH, Monte E. (2012) Phytochrome-imposed oscillations in PIF3 protein abundance regulate hypocotyl growth under diurnal light/dark conditions in Arabidopsis. Plant J 71: 390–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soy J, Leivar P, Monte E. (January 13, 2014) PIF1 promotes phytochrome-regulated growth under photoperiodic conditions in Arabidopsis together with PIF3, PIF4, and PIF5. J Exp Bot http:// dx.doi.org/10.1093/jxb/ert465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YS, Lagarias JC. (2007) Light-independent phytochrome signaling mediated by dominant GAF domain tyrosine mutants of Arabidopsis phytochromes in transgenic plants. Plant Cell 19: 2124–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulijasz AT, Vierstra RD. (2011) Phytochrome structure and photochemistry: recent advances toward a complete molecular picture. Curr Opin Plant Biol 14: 498–506 [DOI] [PubMed] [Google Scholar]
- Van Buskirk EK, Decker PV, Chen M. (2012) Photobodies in light signaling. Plant Physiol 158: 52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N, Kwok SF, von Arnim AG, Lee A, McNellis TW, Piekos B, Deng XW. (1994) Arabidopsis COP8, COP10, and COP11 genes are involved in repression of photomorphogenic development in darkness. Plant Cell 6: 629–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R, Nakamura M, Mochizuki N, Kay SA, Nagatani A. (1999) Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J Cell Biol 145: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Stankey RJ, Vierstra RD. (2013) Structure-guided engineering of plant phytochrome B with altered photochemistry and light signaling. Plant Physiol 161: 1445–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.