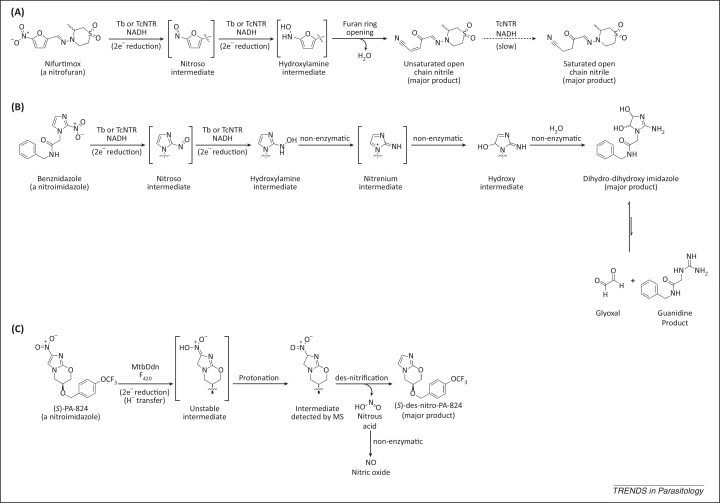
Figure 2.
Mechanism of nitroreductase (NTR)-mediated bioactivation of nitroaromatic compounds. (A) Nifurtimox undergoes two sequential NTR-catalyzed reductions, followed by ring opening to give a toxic unsaturated open-chain nitrile. Tb, Trypanosoma brucei; Tc, Trypanosoma cruzi. (B) The nitro group of benznidazole is reduced to the hydroxylamine by NTR. Subsequently, a series of non-enzymatic transformations leads to a dihydro-dihydroxy product, which can release glyoxal. (C) Unusually, MtbDdn [deazaflavin (F420)-dependent nitroreductase (Ddn) of Mycobacterium tuberculosis (Mtb)] does not reduce the nitro group of (S)-PA-824. Instead, the first step of the bioactivation involves reduction of the C2–C3 bond of the bicyclic imidazooxazine ring system. The unstable product of this reduction is protonated and then undergoes a des-nitrification reaction to give des-nitro-PA-824, with the concomitant production of nitrous acid. Some structures have been abbreviated for clarity. Compounds in square brackets are not experimentally detected. Adapted from [9,61].