Abstract
Experimental studies on traditions in animals have focused almost entirely on the initial transmission phase in captive populations. We conducted an open diffusion field experiment with 13 groups of wild common marmosets, Callithrix jacchus. Seven groups contained individuals that were already familiar with the task (‘push or pull’ box) and thus served as potential models for naïve individuals. Additionally, in four groups one individual was trained for one of the two possible techniques and in two control groups no skilled individuals were present. First, we investigated whether experienced individuals would remember how to solve the task even after 2 years without exposure and whether they would still prefer their learned technique. Second, we tested whether naïve individuals would learn socially from their skilled family members and, more importantly, whether they would use the same technique. Third, we conducted several test blocks to see whether the individual and/or group behaviour would persist over time. Our results show that wild common marmosets were able to memorize, learn socially and maintain preferences of foraging techniques. This field experiment thus reveals a promising approach to studying social learning in the wild and provides the basis for long-term studies on tradition formation.
Keywords: common marmoset, field experiment, memory, persistence, social learning, tradition
Highlights
-
•
We show all key components of behavioural traditions in free-living primates.
-
•
Wild marmosets maintained a foraging technique for over 2 years without exposure.
-
•
Naïve individuals adopted the technique from their skilled family members.
-
•
They preserved their learned foraging variants for at least 9 months.
One of the defining features of human societies is the sophistication in social information transmission, resulting in the accumulation of behavioural traditions and its adaptive modification over time (Tomasello, Carpenter, Call, Behne, & Moll, 2005). Representing a nongenetic inheritance system, this form of social or cultural information transmission has major evolutionary implications (Whiten, 2005): it not only allows the rapid spread of adaptive information through groups, but may also modify selective pressures acting on populations and therefore may influence genetic evolution (Boyd & Richerson, 1985, 2005; Laland, Odling-Smee, & Feldman, 2000).
In the last few decades, several nonhuman animals have been found to be capable of socially transmitting information (Galef, 1976; Heyes, 1994; Laland, 2004; Price & Whiten, 2012; Whiten & Mesoudi, 2008). The first block of findings come from laboratory studies, using a dyadic setting in which one trained model demonstrates a specific behaviour and a single naïve individual (usually termed observer) is allowed to watch, and learn, from the demonstrations (Bugnyar & Huber, 1997; Campbell, Heyes, & Goldsmith, 1999; Galef, Manzig, & Field, 1986; Heyes & Dawson, 1990; Voelkl & Huber, 2000). The focus of these studies is centred on differentiating between possible underlying learning mechanisms, such as enhancement, observational conditioning, imitation or emulation. More recently, studies have tested directly for information transmission in captive social groups by seeding alternative behavioural patterns in different sub-/groups and observing the spread of these patterns (Bonnie, Horner, Whiten, & de Waal, 2007; Crast, Hardy, & Fragaszy, 2010; Dindo, Whiten, & de Waal, 2009; Hopper et al., 2007; Whiten et al., 2007). Although these studies experimentally show the formation of traditions, they are constrained by various factors of captivity and only roughly simulate the species' social structure under field conditions (Galef, 2004; Kendal, Galef, & van Schaik, 2010).
Field studies using the ‘ethnographic approach’ on nonhuman animals show population differences in various contexts that are considered to be independent of genetic and ecological influences (Leca, Huffman, & Gunst, 2007; Ottoni & Izar, 2008; Perry et al., 2003; Rendell & Whitehead, 2001; van Schaik et al., 2003; Whiten et al., 1999). However, since the origin of these differences is often unclear, it is difficult to determine the role of social learning in establishing the behavioural variants (Galef, 2004; Laland & Janik, 2006). Recently, attempts have been made to bridge the gap between the population-level studies under naturalistic settings and the controlled experimental designs in captivity (Reader & Biro, 2010; Whiten & Mesoudi, 2008), testing social/cultural information transmission in free-living animals via so-called ‘open diffusion experiments’ in which a certain behaviour is experimentally seeded into groups and the spread is tracked and recorded (Kendal, Custance, et al., 2010; Schnoell & Fichtel, 2012; Thornton & Malapert, 2009a; van de Waal & Bshary, 2011; van de Waal, Claidière, & Whiten, 2013).
The majority of experimental studies on cultural transmission have focused on the transfer of information, whereas questions concerning the maintenance of the socially acquired information have received limited attention (Hopper, Schapiro, Lambeth, & Brosnan, 2011; Lindeyer & Reader, 2010; Pesendorfer et al., 2009; Thornton, Samson, & Clutton-Brock, 2010). Most of our knowledge come from mathematical models that simulate and/or analyse transmission patterns over several generations and/or investigate the interplay of several factors such as task affordances, social dynamics and memory capacities for the establishment of new behavioural variants in social groups or subgroups (Acerbi, Jacquet, & Tennie, 2012; Allen, Weinrich, Hoppitt, & Rendell, 2013; Claidière & Sperber, 2010; Claidière & Whiten, 2012; Franz & Matthews, 2010; Franz & Nunn, 2009; Hoppitt, Kandler, Kendal, & Laland, 2010; Hoppitt & Laland, 2011; Kendal, Kendal, Hoppitt, & Laland, 2009). For the stability of behavioural variants within a group, conformity (Efferson, Lalive, Richerson, McElreath, & Lubell, 2008) and conservatism/habit formation (Pesendorfer et al., 2009) have been proposed as potential mechanisms. The former has received support mainly from two studies on captive groups of chimpanzees, Pan troglodytes (Hopper et al., 2011; Whiten, Horner, & de Waal, 2005) and one study on wild vervet monkeys, Chlorocebus pygerythrus (van de Waal, Borgeaud, & Whiten, 2013); the latter has been shown in two studies on captive chimpanzees (Hrubesch, Preuschoft, & van Schaik, 2009; Marshall-Pescini & Whiten, 2008) and in wild groups of common marmosets, Callithrix jacchus (Pesendorfer et al., 2009) and may be characterized by a high likelihood of being ‘washed out’ after a short time period (Thornton & Malapert, 2009b). To our knowledge, there are hardly any experimental tests on the stability of socially learned behaviours over a time span longer than a few months.
We here investigated the formation and persistence of an experimentally introduced foraging tradition in common marmosets under natural conditions. We took advantage of the study by Pesendorfer et al. (2009), in which two alternative behavioural patterns were established in family groups of wild marmosets by using an artificial fruit apparatus (push-or-pull box, Bugnyar & Huber, 1997). Note that this initial study was not designed to test for social learning but for the maintenance of initial personal preferences in a group setting. However, it resulted in the majority of monkeys per family group preferring one over the other technique. In the present study, we made use of this situation and tested (1) the long-term memory of experienced monkeys, (2) the information transmission from experienced to naïve group members (that have been born and/or immigrated in the 2 years since the original study) and (3) the persistence of socially learned techniques over several test blocks conducted in the course of 9 months. We expected (1) experienced individuals to remember the task and show a preference for their previously learned technique and (2) naïve individuals to learn socially from skilled family members to solve the task using the same technique. Concerning (3), we did not have a clear expectation since the learned behaviours might persist over time or collapse at a certain point, that is, the preference for a technique would fade and the distribution of both alternative techniques become random.
In addition to the groups used in the Pesendorfer et al. study (2009), we incorporated six further groups. In four of the groups we trained one individual on one of the two possible techniques (two individuals learned to pull and two to push). This allowed us to see whether the presence of just one skilled individual would be sufficient to get the transmission going and establish a group ‘norm’ and, in comparison to groups with experienced subjects from the previous study, whether recently trained individuals would use the trained technique more reliably than those relying on long-term memory. Furthermore, two other (untrained) groups served as additional controls.
Methods
Study Site and Population
The study was conducted between September 2009 and May 2010 on wild common marmosets in an area of 32 ha, part of a ca. 100 ha fragment of mixed primary and secondary Atlantic Forest, 40 km west of Recife in the state of Pernambuco, northeast Brazil (see Souto, Bezerra, Schiel, & Huber, 2007 for a description of the study site).
Thirteen family groups (comprising 4–15 individuals each) participated in our experiments (Table 1). Six groups were naïve to the task, but seven groups contained individuals that participated in the study by Pesendorfer et al. (2009) and were therefore already familiar with this experiment (henceforth ‘experienced groups’). Nevertheless, in the meantime (the time span without exposure to the task was about 2 years) the composition of these groups had changed because of births, deaths and individuals dispersing to other family groups. Hence, these groups also included naïve individuals (see section Experimental Conditions for further details). All subjects could be identified individually (see the Appendix and Schiel, Souto, Bezerra, & Huber, 2008 for procedure) and were assigned to four different age categories (adults/subadults: >11 months; juveniles: 5–10 months; older infants: 3–4 months; young infants: 0–2 months; Schiel & Huber, 2006). For detailed group compositions see Table A1. This study complied with Brazilian law.
Table 1.
Number of individuals per group participating in the pull, push and free conditions
| Condition |
Pull |
Push |
Free |
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | A* | T* | C | P | L* | W* | B | E | F* | H* | S* | G | K | |
| N (total) | 9 | 12 | 9 | 8 | 8 | 4 | 15 | 8 | 11 | 8 | 6 | 6 | 7 | 111 |
| N (skilled) | 6 | 3 | 1 | 1 | 4 | 3 | 2 | 1 | 4 | 3 | 1 | 0 | 0 | 29 |
| % Skilled | 66.7 | 25 | 11.1 | 12.5 | 50 | 75 | 13.3 | 12.5 | 36.4 | 37.5 | 16.6 | 0 | 0 | 26.1 |
| Attempts±SE | 126.4±33.9 | 122.2±30.7 | 107.0±27.5 | 186.1±38.9 | 129.1±22.0 | 122.3±29.9 | 117.9±29.0 | 98.0±26.3 | 151.4±39.2 | 105.5±37.2 | 111.2±23.8 | 45.7±21.3 | 147.0±56.2 | |
Groups indicated by asterisks contained several potential models (N skilled), because they had already participated in the pilot study in 2007 (Pesendorfer et al., 2009). In the other groups a model was newly trained (condition pull or push) or none of the subjects received specific training (condition free). The mean number of all push and pull attempts ± SE for each family group are also included.
Apparatus
We used the same apparatus as in the study by Pesendorfer et al. (2009), a replica of the push-or-pull box (20 × 10 × 10 cm) designed by Bugnyar & Huber (1997). Prior to the experiments, all family groups were habituated to the experimental set-up by food provisioning (apples and bananas). The wooden box could be manipulated in two different ways, by either pushing or pulling an opaque flap door on one side to gain access to the rewards inside (Fig. 1a, b). Details of the experimental set-up are described in the Appendix (see also Fig. A1).
Figure 1.
Apparatus. An individual could either (a) pull or (b) push the flap door to gain access to the rewards inside the wooden box.
Experimental Conditions
Depending on their condition in the pilot study (Pesendorfer et al., 2009), the experienced groups were divided into three corresponding experimental conditions, namely pull (groups A and T), push (groups L and W) and free condition (groups F, H and S; see Table 1). Importantly, although the free condition groups included individuals with preferences for either technique, tendencies for one favoured technique could be observed at the group level. In addition, in four of the six new groups we trained one dominant individual (always the dominant male, except for group P) to perform the pull or the push technique (pull: C and P; push: B and E; the conditions were randomly chosen). The training phase of the model lasted for 10 sessions (one session per day with 10 trials each) consisting of 100 demonstration trials in sum (see the Appendix for details of the training procedure). Note that in the experienced groups no individuals were trained, but that in these groups the experienced individuals served as potential models for the naïve subjects in their group. Experienced subjects could thus remember their preference, but naïve subjects had to learn the technique. The two remaining groups (G and K) served as additional control groups with no experienced or trained individuals. However, there are potential ‘models’ in all of these groups, since any manipulating individual might serve as a model to an observer. We argue that the crucial difference is the ‘training status’ of the models. Whereas experienced individuals were trained 2 years beforehand, the models in the four additional groups were trained recently and the models in the control groups (i.e. the first individuals that manipulated the apparatus) were not trained at all.
Procedure and Data Collection
The test procedure was the same for all groups. We conducted three test blocks over a period of 9 months (with a break of 52 days between each block), each consisting of 10 test sessions with three trials each (in sum 90 test trials per group were conducted). Group G dispersed in the last break and so only two test blocks could be conducted for this group. The number of rewards in the box was equivalent to the number of individuals in the group, so that each member had the same potential chance to get a reward in each trial. In the rare event (1.67% of all occasions) where a marmoset managed to take more than one reward at once, we rebaited the box at the end of this trial with the respective number of additional rewards. Experiments started only if at least 75% of all group members were present (defined as being present within a radius of 10 m of the experimental set-up). Before the test session started, the apparatus was positioned on the platform and covered with a cloth. After the box was baited with the respective number of rewards (banana pieces, approximately 1 × 1 cm) the cloth was removed and the animals were allowed to approach the box. The animals could freely manipulate the box and always open it in two different ways (by either pulling or pushing the door). After a trial was finished and the box was empty, the experimenter covered it and refilled it with rewards. The time between trials was about 2 min. No more than one session (= 3 trials) per group was conducted per day (with no more than 1–3 days between sessions of one test block (= 10 sessions)). All training and test sessions were filmed with a digital video camera (Sony DCR-SR35, Hybrid HDD) and continuous comments about the identity and actions of all visible individuals (manipulators, bystanders within 30 cm of the box, observers, scroungers and other animals) were recorded.
The participation of all groups was high throughout the experiment (92.22% of all possible group members participated; see Table 1 for the mean number of all push and pull attempts at the group level). In each family group naïve subjects were also present (mean number of naïve individuals/group = 6.16 individuals; range 1–13) and participated (90% of naïve individuals participated/ group; range 1–11; see Appendix Table A2 for the mean proportion of push attempts at the individual level). Note that owing to the group setting in a natural environment there is a high variability of exposure to the task. Some individuals were already present at the first test session whereas other subjects were just born later during the course of experiments.
Data Coding and Analysis
All training and test trials were coded in a frame by frame analysis using Adobe Premiere Pro CS4. The following parameters were recorded from each trial: (1) the duration of each trial, (2) the identity of each subject that was present (within a radius of 10 m of the experimental set-up), (3) the identity of the subject manipulating the box, (4) the technique (‘push’ or ‘pull’) and number of actions performed by the subject (defined as door movements from the neutral vertical position), (5) the number of successful openings and (6) the number of rewards gained. For reliability purposes a second coder blind to the experimental conditions coded 100 randomly selected subtrials. Interobserver reliability was excellent for the type of technique used (Cohen's kappa = 1.0) and very high for the number of manipulations (Cohen's kappa = 0.96).
Owing to variation in sample size and deviation from normal distribution, nonparametric analyses were conducted, using IBM SPSS Statistics version 20. All analyses were two tailed and P values ≤0.05 were considered as statistically significant, and P values >0.05 and ≤0.1 as trends. We used binomial generalized linear mixed models (GLMMs) with a logit link function, with either technique (pull/push) or success (yes/no) as the dependent variable and several fixed predictor variables, depending on the purpose of the model (see Results for details). To control for pseudoreplication, individual and group identity were included as random factors in all models. We used a stepwise procedure of selection of variables, using Akaike's information criterion (AIC) to compare models and as the basis for our selection of the best model. For clarity, we present only the best-fitting models with all significant effects in the Results. The significant and nonsignificant results for the fixed factors, as well as the coefficient ± SE for the significant factors for each model, are provided in the Appendix tables.
Results
Memory
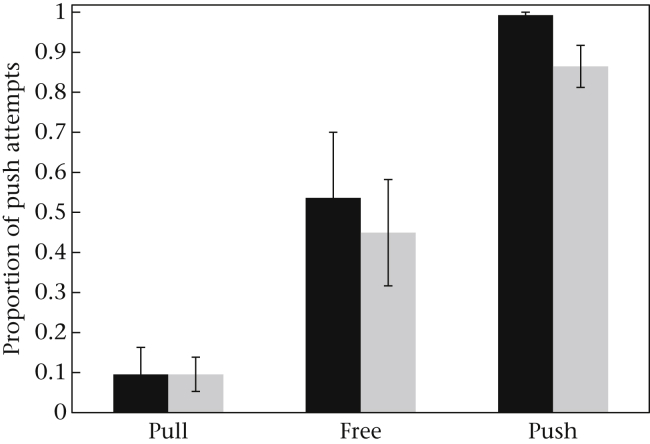
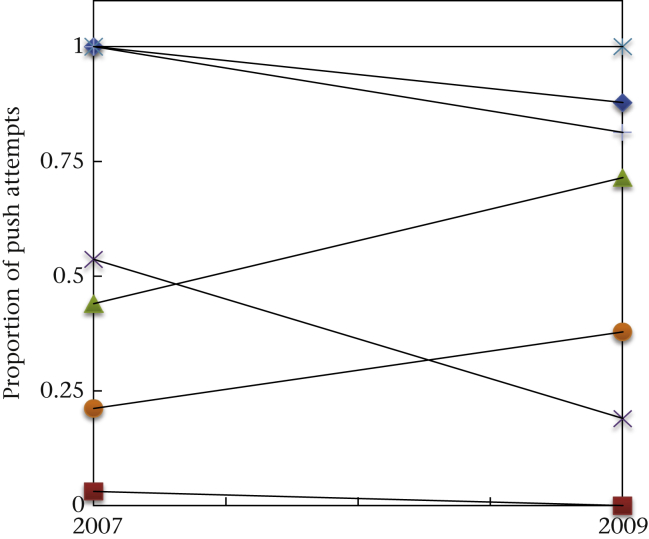
We identified 24 individuals from seven groups that had taken part in the initial study in 2007 (Pesendorfer et al., 2009). All except one of these experienced individuals readily participated in this experiment again. To assess whether these individuals remembered the technique they had learned in 2007, we calculated a binomial GLMM with the technique (push/pull) as the dependent variable and the following fixed factors: time (last six sessions of 2007 versus first six sessions of 2009), condition (push, pull or free) and sex. The factor ‘sex’ was included to investigate possible differences between males and females (see Box, Yamamoto, & Lopes, 1999). Age was not included since all experienced individuals belonged to the same age class (i.e. adults). The model showed a significant effect of condition, but also of time (Fig. 2, Appendix Table A3). A significant interaction between condition and time (Appendix Table A3) showed that the marmosets pulled slightly more in 2009 than in 2007. This is especially true for the individuals in the push and free conditions (see Fig. 2). Despite this effect, there was still a significant influence of condition, that is, experienced individuals performed their previously preferred technique: those that were pulling 2 years before still preferred pulling and those that were pushing preferred pushing. These findings were corroborated when we calculated binomial tests for each individual (which showed more than six manipulations during the first six sessions in 2009): the preferences were significant for 18 of 20 monkeys (binomial test: P < 0.05); the two subjects that had no preference in 2009 did not have a preference in 2007 either. Note that monkeys from the free condition were not trained on a particular method, but did develop individual preferences in the course of the initial study, which also remained in the current study (as the model takes individual into account, this is not merely a by-product of mean performances; see Appendix Fig. A2). Four individuals that were present but did not manipulate the apparatus during the study in 2007 at all, but participated in 2009, all used the technique trained or preferred in their family group.
Figure 2.
Mean ± SEM performance of experienced individuals from all three conditions (pull, free and push) in 2007 (black bars) and 2009 (grey bars).
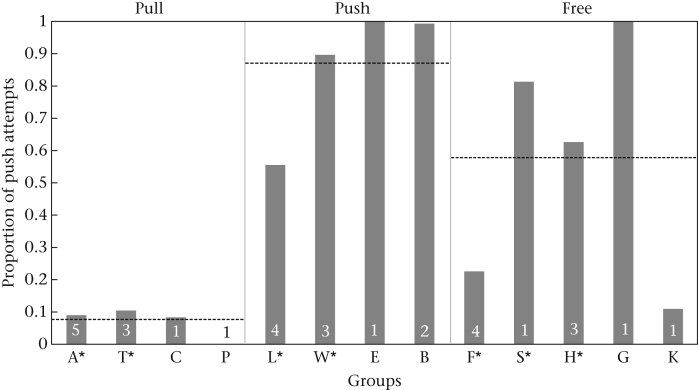
To clarify how well the monkeys remembered the task, we compared the manipulations (push/pull) of the already experienced individuals (from 2007) and the recently trained subjects (from 2009) of the pull and push condition during their first test block. We included the following fixed factors in the binomial GLMM: year (2007 versus 2009), condition (push versus pull) and sex. Age was not included since all experienced and freshly trained individuals belonged to the same age class (i.e. adults). The results confirmed that only the condition significantly explained the variance and that there was no significant difference in the performance (i.e. the dependent variable) between ‘previously trained’ and ‘recently trained’ individuals of the pull and push conditions (Fig. 3, Appendix Table A4).
Figure 3.
Mean proportion of push attempts of models (experienced and trained) for each group and condition. The dotted lines represent the mean proportion over all groups in this condition and the numbers indicate the sample size of models per group. Asterisks indicate groups with experienced models that participated in the pilot study in 2007 (Pesendorfer et al., 2009).
In the free condition of the initial study, one group (F) developed a preference for pulling and two groups (H and S) a preference for pushing (Fig. 3). Hence, all of these experienced individuals could serve as models for naïve animals in the current study, although they were not explicitly trained in the initial study. The same logic applied to two subjects of group G and K that instantly learned to solve the task in the free condition in 2009: in both cases the dominant female found out how to manipulate the box successfully by using one of the two techniques (in group G, GAB with push and in group K, KAT with pull) and continued to do so throughout the experiment. Consequently, we treated experienced individuals from groups F and K as potential demonstrators for pulling and those from groups H, S and G as potential demonstrators for pushing, but analysed them separately from groups with trained models in pulling and pushing.
Transmission
To test whether naïve individuals adopted the same technique as predominantly used in their family group we calculated a binomial GLMM and included the technique (push/pull) of each naïve individual as the dependent variable and the following fixed factors: condition (push, pull, free push or free pull), sex, age (juvenile versus adult), year (2007 versus 2009) and presence (present in at least seven of the first 10 sessions versus not present in more than three of the first 10 sessions). The factor ‘age’ was included to assess whether juveniles and adults would differ in learning/adopting a technique shown by an adult model and the factor ‘sex’ to test for possible sex differences. To see whether the ‘training status’ of the models would have an influence, we added the factor ‘year’ (2007 with experienced individuals versus 2009 with recently trained individuals). The factor ‘presence’ was included as a proxy of how often family members were in seeing distance compared with just in hearing distance. The underlying logic was that if naïve individuals were often out of sight of most group members (i.e. showing a low degree of social proximity and hardly ever ‘present’ at the experimental set-up), they would lack opportunities to learn from skilled conspecifics. In this respect, the initial phase (first test block = first 10 sessions) was considered crucial because at that time mainly skilled individuals were manipulating the apparatus efficiently and consistently with one technique (i.e. being rewarded for their pulling and pushing attempts, respectively); later on more and more (originally naïve) subjects gave it a try themselves, resulting in a greater variety of actions that could or could not lead to success. We wanted to examine whether the presence of naïve individuals in this crucial initial phase would have a significant influence on whether or not they would adopt the technique and thus added the factor ‘presence’ as an estimated measure for social proximity (i.e. being in seeing distance).
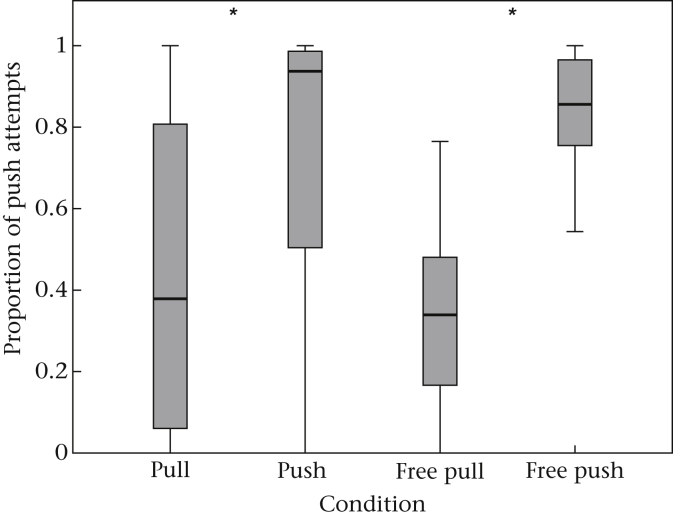
We found a significant interaction effect of condition with the factor presence (Appendix Table A5), showing that those individuals that were present during the initial phase (first 10 sessions) also learned best. Additionally, there was an age effect (Appendix Table A5), showing that young naïve monkeys were pushing more often in the pull condition than adults. Owing to the clear effect of presence that is connected to the opportunity to learn from skilled individuals, we ran the model again but excluded those individuals that were by definition ‘not present’ during the crucial initial phase. As expected, the factor condition was significant, indicating that naïve individuals that experienced group members using predominantly the pull method tended to use pulling themselves, whereas those that experienced group members using predominantly the push method tended to use pushing (Appendix Table A5). This was true not only when subjects had access to trained models but also when they had access to skilled subjects in the free condition, and the performance of the mutual free and model conditions (i.e. free pull versus pull; free push versus push) did not differ significantly (Fig. 4, Appendix Table A5). The age effect was again significant (Appendix Table A5).
Figure 4.
Mean proportion of push attempts of naïve subjects in the pull and push and the free pull and free push conditions. The box plots show the median and 25th and 75th percentiles; the whiskers indicate the values within 1.5 times the interquartile range. *P < 0.05.
To clarify how stable the (originally naïve) individuals performed the learned techniques, we ran a similar binomial GLMM with all the previous factors, but compared the manipulations of the naïve individuals with those of the experienced individuals. Therefore, we included ‘experience status’ (experienced/trained versus naïve) as an additional fixed factor. Here, to make a fair comparison, we again excluded the nonpresent naïve individuals. As before, the best-fitting model revealed a significant effect of condition and an age effect (Appendix Table A6). Additionally, a significant interaction of condition and age (Appendix Table A6) showed that young individuals in the pull condition tended to push more often than adults. Nevertheless, we did not find a significant difference between experienced/trained and naïve individuals, indicating that they performed in a similar way.
Finally, we examined the success of performing the learned techniques, focusing on those cases when the manipulation of the door resulted in retrieving a reward. Therefore, we ran a binomial GLMM with technique (push/pull) as the dependent variable, to assess which factors influence which type of technique (leading to the reward) was used. As previously, we again included condition (push, pull, free push, free pull), sex, age (juvenile versus adult), year (2007 versus 2009) and presence (present versus not present during initial phase) as the fixed factors. The model again showed a significant interaction effect between condition and presence and a significant effect of age class, with juvenile monkeys being more often successful with the push than the pull technique regardless of condition (Appendix Table A7). After the exclusion of the nonpresent individuals, the best-fitting model showed, as expected, a significant condition effect and again the age class effect (Appendix Table A7).
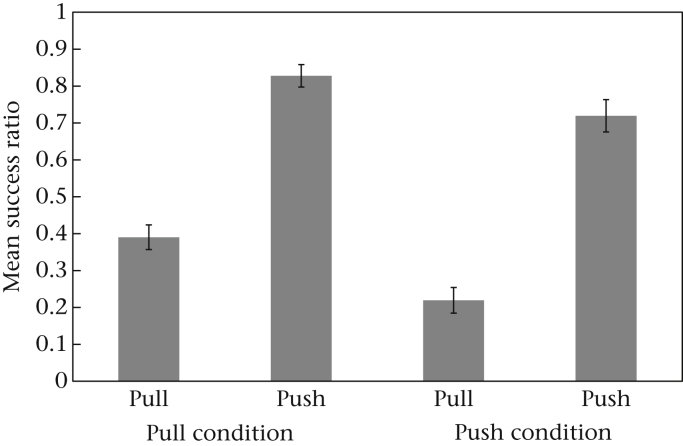
Since young monkeys appeared to find one technique easier than the other, we analysed whether the two opening techniques differed in efficiency in general (for adults and juveniles). Therefore, we ran a binomial GLMM to test which variables could predict whether an action would be successful or not (1/0) and included the following fixed factors: technique used (pull or push), age (juveniles versus adults), sex and test block (1, 2 or 3). The factor ‘condition’ was excluded because we had already showed that individuals predominantly used the technique corresponding to their condition. We included the factor ‘test block’ because we expected individuals would become more efficient over time. The best-fitting model confirmed that pushing was the more efficient technique compared to pulling, as we found a significant effect of technique used (Appendix Table A8). Note that this was true even for those individuals that preferred pulling (Fig. 5). The factor age again had a significant influence, with young individuals being less efficient than adults. The model also showed that overall individuals became more efficient over time (Appendix Table A8). An interaction effect between test block and technique showed that the animals became more efficient with pushing after the first test block whereas the efficiency of pulling remained constant over the different test blocks (Appendix Table A8).
Figure 5.
Mean ± SEM success ratio of both techniques (push and pull) for individuals that belonged to either the pull or push conditions.
Persistence
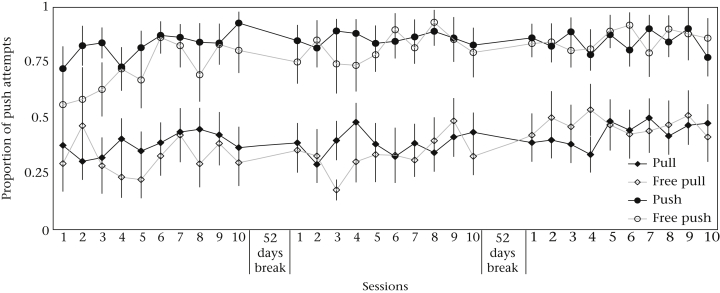
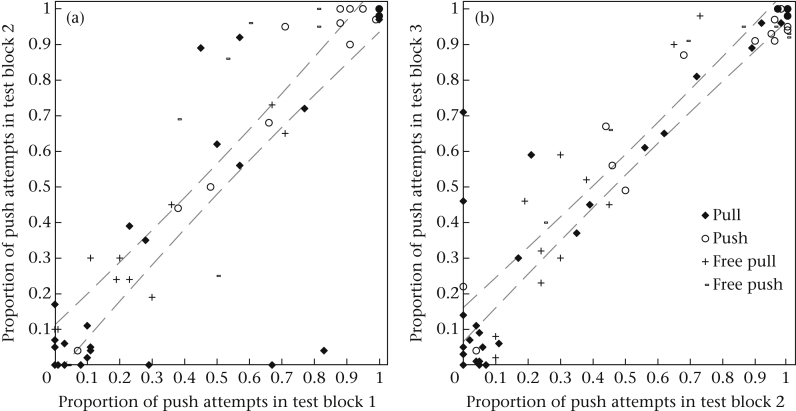
Given that the push technique was more efficient than pulling, it was of particular interest whether the performance of the subjects was consistent over time or would converge to pushing in the course of several test blocks. To assess the repeatability across the experimental test blocks, we calculated the intraclass correlation coefficient (ICC). This assessed the proportion of variation in behaviour that was due to interindividual versus intraindividual variation. The results showed that the overall performance was highly consistent over time (Cronbach’s alpha = 0.965, N = 3, ICC = 0.902, P < 0.001; Fig. 6, Appendix Fig. A3).
Figure 6.
Mean ± SEM proportion of push attempts per condition over all three test blocks (= 30 sessions).
To examine whether the persistence of the techniques used differed between conditions, we calculated a binomial GLMM with technique (push/pull) for each individual as the dependent variable and the following fixed factors: session number (1–30), condition (pull, push, free pull, free push), age (juveniles versus adults) and sex. As expected, the model showed a clear condition effect (Appendix Table A9). There was a significant positive effect of session number, confirming that individuals tended to push more often over time, especially in the pull condition as there was an interaction effect (Appendix Table A9). In addition, age again had a significant effect, with young subjects pushing more often than adults in the pull condition (Appendix Table A9). Hence, individuals did modify their preferences over the course of test blocks, but only to a relatively ‘mild’ extent because their overall performance was not affected (Fig. 6).
Discussion
Wild marmosets were able to maintain a preference for one of two opening techniques at an artificial fruit apparatus over a 2-year period without exposure to the task. Furthermore, naïve conspecifics adopted a preference for the particular opening technique used by skilled subjects of their group and these socially learned preferences remained stable over a period of several months during which subjects had repeated exposure to the task. We thus show, to our knowledge for the first time, all three key components for behavioural traditions of alternative foraging techniques under field conditions, namely memory for learned behavioural variants, social transmission of these variants to naïve conspecifics (horizontally and vertically) and persistence of these variants over time (compare Fragaszy & Perry, 2003; see for another recent example, although on food preferences and only over a period of a few months, van de Waal, Borgeaud, et al., 2013).
Memory
Individuals that had experience with pulling or pushing a door of an artificial fruit apparatus in the study by Pesendorfer et al. (2009) instantly opened the apparatus again after 2 years without exposure. Notably, they consistently applied their previously learned technique, performing almost identically to freshly trained models. These findings provide a clear demonstration for long-term memory of particular foraging techniques in wild marmosets. Furthermore, they indicate that group-specific differences in foraging styles may remain stable even though they cannot be practised for some years. Memory is a crucial aspect since a tradition has to be persistent over time, not only when the behaviour can be executed regularly, but especially also after times where no opportunities for practice were given, for instance when food sources (e.g. fruits, insects) are irregular in time and/or location. In this respect, our results are in line with findings on Japanese macaques, Macaca fuscata, which kept performing given behaviours over some years without having frequent/unlimited exposure to the respective food sources (Huffman & Hirata, 2003). Still, we are aware of hardly any (laboratory and field) studies on primates testing for long-term memory of experimentally induced skills under such standardized conditions as in the current study (but see Whiten et al., 2005).
Transmission
Importantly, the majority of individuals in the study groups had not participated in the experiment by Pesendorfer et al. (2009) because they had recently immigrated or were born in the last 2 years, whereas others had disappeared or died. That these experimentally naïve subjects adopted the same technique as their experienced group members makes a strong case for social learning and information transmission, horizontally to immigrants and vertically to offspring, respectively. Note that we do not make any claim about the exact learning mechanism (Heyes, 1994; Zentall, 2006), although the design of the two-action apparatus (bidirectional control) makes higher forms than enhancement and social facilitation (i.e. above the perception and motivation level) likely (Heyes & Dawson, 1990; Miller, Rayburn-Reeves, & Zentall, 2009; Tennie, Call, & Tomasello, 2006). In line with studies from captivity (Bugnyar & Huber, 1997; Burkart, Fehr, Efferson, & van Schaik, 2007; Caldwell & Whiten, 2003; Voelkl & Huber, 2000), experimentally naïve marmosets showed high levels of social proximity to skilled conspecifics (i.e. were often present during the experiment and in close seeing distance to their family), which in turn appeared to be relatively tolerant to group members at the apparatus (Gunhold, Massen, Schiel, Souto, & Bugnyar, n.d.). That those subjects that were most often seen together with skilled models learned best further supports the notion of social information transmission and fits well to the ideas of Coussi-Korbel and Fragaszy (1995) concerning the interplay of social learning and social dynamics.
The resulting group-specific preferences in using one over the other opening technique cannot be explained by a general preference for one of the two techniques by marmosets. From the five groups of the free condition (in which no model was explicitly trained and both opening possibilities were available from the onset of the studies), three groups ended up with a preference for pushing and two with a preference for pulling. The performance of individuals in these free push and free pull groups did not differ from that of members of the push and pull groups with trained models. Although these results suggest that marmosets use both techniques roughly with the same probability, it could be argued that the pull technique is more demanding since it requires holding the door open with one hand while grasping for the food (Bugnyar & Huber, 1997). Owing to their small body size and little strength, this would be especially true for young monkeys. The observed differences in manipulation attempts between adults and juveniles in the pull condition may hint in this direction.
When analysing the relative success of the opening attempts, pushing clearly comes out as the more profitable technique: across conditions, monkeys got a piece of food in almost every pushing attempt, whereas they were successful in only about every second pulling attempt. Subjects from the pull groups became more efficient than subjects from the push groups in either technique, pulling and pushing. Possibly, the difficulty in succeeding with pulling affected the monkeys' motivation and/or concentration in performing the task.
Persistence
There is a crucial difference between the ‘memory’ and ‘persistence’ of the foraging techniques in respect of the exposure to the task. While we could show that the marmosets remembered the technique over a gap of 2 years without having access to the apparatus, it is essential that the subjects have repeated access to the task when examining the persistence of a preferred technique over a longer time period. Our results revealed that marmosets were capable of ‘preserving’ their socially learned foraging variants for at least 9 months, despite having repeated access to the test apparatus and, thus, ample opportunities to execute, and modify, their behaviour. Indeed, hardly any subject used pulling or pushing exclusively, indicating that they did had some experience with both opening techniques. Given that the two techniques differ in effort and efficiency, it is surprising that subjects of the pull groups hardly ever changed their preferences towards the more easy and profitable pushing.
Claidière and Sperber (2010) argued that if two possible solutions are available to solve a problem and the subjects acquire one solution by social learning, then the preference for this solution should collapse over time if there are no stability mechanisms. One of the main stability mechanisms is ‘conformity’, where individuals are adapting their behaviour to the majority of the group (Claidière, Bowler, & Whiten, 2012; Claidière & Whiten, 2012; Lachlan, Janik, & Slater, 2004). For example, Whiten et al. (2005) showed that captive chimpanzees that discovered an alternative method in a tool use task continued to match the predominant approach of their group members and thus showed a ‘conformity bias’. Alternatively, habit formation has been proposed to explain group-specific preferences for foraging techniques (Hrubesch et al., 2009; Marshall-Pescini & Whiten, 2008). In the initial study on marmosets, for instance, individuals of a group learned one solution during training and kept their individual habits even when an alternative method became available (Pesendorfer et al., 2009).
It is possible that a similar process is responsible for some of our current results: once learned, individuals appeared to get stuck with a given method. However, unlike in Pesendorfer et al. (2009), marmosets always had both opening options available, making it highly unlikely that several individuals of a group develop a preference for the same technique without any social input. Indeed, as discussed above, the learning process in the current study was probably triggered by observation, indicating that any form of habit formation would thus be based on socially biased (individual) learning (Fragaszy & Visalberghi, 2004).
The high fidelity in performing a particular behaviour (once it has been learned) speaks against a conformity bias, which would require subjects to be flexible enough to converge to a given behaviour that is shown by most of the other group members. The results from the free condition, however, raise the possibility that at the beginning of the experiment social effects may have promoted conformity: although none of the groups had a specifically trained model, in each of the groups individuals converged either to pulling or pushing. Such group preferences could be the result of each individual being socially influenced in the same way by particularly attractive individuals such as the dominant breeding pair. Alternatively, the convergence in the free condition could also be explained by the fact that the first individual that learned to solve the problem served as a model for the others. Thus, this would be similar to the model conditions, but with the difference that these models were not trained or experienced.
In comparison to the social groups of most primates, family groups of callitrichids are structured not so much by kin and friendship relations but by age class and breeding status (Digby, 1995). Still, model identity (and features such as sex, age and breeding status) might have a profound influence on the acquisition and transmission of foraging variants (Horner, Proctor, Bonnie, Whiten, & de Waal, 2010; Laland, 2004; Nicol & Pope, 1999). Notably, there could be more than one transmission line within a family, for example along individuals with a high association index such as same-aged peers (Kendal, Custance, et al., 2010; Müller & Cant, 2010). Recent experimental studies on social learning and tradition formation under field conditions have revealed strong effects of social structure and contextual variables in different species (Biro et al., 2003; Thornton & Clutton-Brock, 2011; van de Waal, Borgeaud, et al., 2013; van de Waal, Bshary, & Whiten, 2014; van de Waal, Renevey, Favre, & Bshary, 2010). Marmosets represent an excellent primate example for cooperatively breeding systems (Tardif, Harrison, & Simek, 1993). Taking into account model identity and subgroup structure in the transmission patterns may be the next steps.
Acknowledgments
This study was supported by FWF (Austrian Science Fund) project Y366-B17 to T.B., a KWA fellowship of the University of Vienna and an IPS research grant to T.G., and a Lise Meitner fellowship of the FWF (M 1351-B17) to J.J.M.M. We thank Ludwig Huber, Friederike Range and Mario B. Pesendorfer for their roles in initiating social learning studies on wild marmosets, and Orlaith Fraser, Markus Böckle, Corsin Müller and Claudia Stephan for constructive feedback on the manuscript and statistical advice. We are also grateful to Vedrana Šlipogor who served as independent coder and to Nichola Raihani, Angela Turner as well as three anonymous referees for useful comments on the manuscript.
Appendix.
Methods
Methods of identification
Similar to the pilot study by Pesendorfer et al. (2009), for each family group a wooden platform was positioned in their home range and the animals were habituated to the experimental set-up by food provisioning (apples and bananas). During this habituation phase, focal and all-animal sampling (Altmann, 1974; Lehner, 1996) were used to identify the composition of each group. Hence, for each individual, an identification profile was constructed (including sex, age class, status, portrait pictures, specific body and facial features, e.g. scars, injuries, etc.; Schiel et al., 2008). If an individual could not be reliably distinguished from the others, it was marked by cutting a little part of the fur on the tail. This procedure was completely noninvasive, since the experimenter (T.G.) did not catch the animal but merely distracted it by simultaneously provisioning food on the platform (no visible effects on the behaviour of the animal were seen afterwards). All the monkeys were named with letter codes and individuals belonging to the same group share the same first letter.
Apparatus and Experimental Set-up
A small lockable door on the top of the box allowed the experimenter to refill the ‘push-or-pull box’ without manipulating the swinging door. As we tested the animals in a group setting, we had to ensure that as few animals as possible could manipulate the apparatus at the same time to facilitate later analysis of the performance. Thus, we attached a wooden frame (50 × 50 cm) around the front end of the box to prevent possible manipulations from the top or the side (Fig. A1). Therefore, the marmosets could only manipulate the flap door while sitting on a small platform (10 × 10 cm) in front of it. The whole apparatus was then mounted and fixed onto the big platform (post: 140 cm high) that was used for the habituation phase and could be reached by using a perch (about 120 cm long) as a bridge from the nearest tree or bush (Fig. A1).
Model training
In group B, an additional male was trained since he immigrated from another group (pull condition) during the course of the experiments. As we worked with free-living groups we could not freely choose a specific individual and train it in visual isolation from the others. Therefore, we offered the dominant individual the baited box (the flap door was removed) and let it feed out of it. Subsequently, the first training session started in which the door was attached again and a small stopper was fixed in place to prevent the animals from adopting the alternative technique. The training session started only when the model was present and not distracted (e.g. while hunting or eating). After we had positioned the apparatus on the platform, in all four groups it was immediately monopolized by the chosen dominant individual. In the cases where the other individual of the dominant pair or other group members tried to reach the box and gain their own personal experience (and the chosen model was not preventing them from doing so) the experimenter approached the set-up and covered the flap door with a wooden plate. Consequently, naïve subjects never managed to interact with the box in its blocked state and therefore did not have the opportunity to learn individually during this period. Apart from that, the experimenter was standing about 2 m from the platform, watching the model manipulating the box. In each session, the box was baited with 10 rewards (banana pieces, 1 × 1 cm) and thus the session ended after 10 demonstrations by the model. If the model took two pieces of food or the reward fell down, the experimenter refilled the box with an additional piece of reward at the end of the session.
Figure A1.
Experimental set-up with test platform, mounted box with shield and perch as bridge for the subjects.
Figure A2.
Mean proportion of push attempts of experienced subjects of the free condition in 2007 (pilot study, Pesendorfer et al., 2009) compared to their performance in 2009 (current study).
Figure A3.
Intraclass correlation coefficient (ICC) scatter plots of the proportion of push attempts for each condition comparing (a) test block 1 with test block 2 and (b) test block 2 with test block 3. The dashed lines indicate the 95% confidence interval.
Table A1.
Composition of family groups
| Group | Adults & subadults | Juveniles | Old infants | Young infants | N | N (max) |
|---|---|---|---|---|---|---|
| A | 7/8 | 1/1 | 1/0 | 0/2 | 9/11 | 11 |
| B | 11/10 | 1/2 | 0/1 | 0/0 | 12/13 | 15 |
| C | 5/6 | 1/3 | 2/0 | 0/0 | 8/9 | 9 |
| E | 3/4 | 1/0 | 1/0 | 0/2 | 5/6 | 8 |
| F | 6/8 | 4/4 | 2/2 | 0/0 | 12/14 | 14 |
| G | 3/4 | 0/1 | 0/0 | 0/0 | 3/5 | 6 |
| H | 4/2 | 0/2 | 2/2 | 0/0 | 6/6 | 8 |
| K | 5/4 | 2/1 | 0/0 | 0/0 | 7/5 | 7 |
| L | 4/5 | 2/2 | 2/1 | 0/0 | 8/8 | 9 |
| P | 5/6 | 2/2 | 2/0 | 0/2 | 9/10 | 10 |
| S | 3/4 | 2/1 | 0/0 | 0/0 | 5/5 | 6 |
| T | 5/6 | 3/4 | 2/2 | 0/0 | 10/12 | 12 |
| W | 3/4 | 1/0 | 0/0 | 0/0 | 4/4 | 4 |
| Total | 64/71 | 20/23 | 14/8 | 0/6 | 98/108 | 119 |
Numbers refer to the beginning and the end of the study. Following Schiel and Huber (2006), we assigned the individuals to four different age categories (adults/subadults: >11 months; juveniles: 5–10 months; older infants: 3–4 months; young infants: 0–2 months). N (max) indicates the total number of the individuals belonging to each group during the whole experiment). Note that the number of monkeys per group varied across the different sessions and test blocks, mainly because of births (>50), deaths, disappearances or emigration to other groups.
Table A2.
Mean proportion of push attempts over the whole study period for all individuals of each family group
| Group | Condition | Individual |
Mean±SE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |||
| A | Pull | 0.044 | 0.025 | 0.006 | 0.634 | 0.807 | 0.305 | 0.232 | 0.611 | 0.333±0.111 | |||||
| B | Push | 1.000 | 1.000 | 0.983 | 0.995 | 0.504 | 0.056 | 0.492 | 0.000 | 1.000 | 0.118 | 0.718 | 0.333 | 0.987 | 0.630±0.111 |
| C | Pull | 0.167 | 0.333 | 0.077 | 0.757 | 0.333 | 0.989 | 0.046 | 0.074 | 0.086 | 0.318±0.113 | ||||
| E | Push | 1.000 | 0.907 | 0.947 | 1.000 | 0.750 | 0.968 | 1.000 | 0.939±0.034 | ||||||
| F | Free pull | 0.765 | 0.000 | 0.051 | 0.329 | 0.757 | 0.455 | 0.167 | 0.481 | 0.981 | 0.213 | 0.035 | 0.385±0.101 | ||
| G | Free push | 1.000 | 0.786 | 0.873 | 1.000 | 0.882 | 0.908±0.041 | ||||||||
| H | Free push | 0.776 | 1.000 | 1.000 | 0.967 | 0.413 | 0.610 | 0.794±0.099 | |||||||
| K | Free pull | 0.000 | 0.339 | 0.247 | 0.280 | 0.410 | 0.000 | 0.500 | 0.254±0.073 | ||||||
| L | Push | 0.986 | 0.833 | 0.965 | 0.964 | 0.500 | 0.537 | 0.222 | 0.715±0.113 | ||||||
| P | Pull | 0.576 | 0.000 | 0.387 | 0.043 | 0.019 | 0.061 | 0.342 | 0.204±0.086 | ||||||
| S | Free push | 0.042 | 0.544 | 0.897 | 0.839 | 0.734 | 0.963 | 0.670±0.139 | |||||||
| T | Pull | 0.977 | 0.044 | 0.991 | 0.370 | 0.000 | 0.071 | 0.720 | 0.063 | 0.097 | 1.000 | 0.048 | 1.000 | 0.448±0.129 | |
| W | Push | 0.910 | 0.937 | 0.333 | 0.950 | 0.783±0.150 | |||||||||
Experienced individuals are in bold; freshly trained individuals are in italic; naïve individuals are not specifically indicated.
Table A3.
Memory
| Variable | F | df | Coefficient±SE | P |
|---|---|---|---|---|
| Time | 10.123 | 1 | 0.001 | |
| Condition | 21.255 | 2 | <0.001 | |
| Sex | 2.961 | 1 | 0.085 | |
| Condition*Time | 5.781 | 2 | 0.003 | |
| Condition*Sex | 2.583 | 2 | 0.076 | |
| Time=2007 | −0.207±0.165 | 0.210 | ||
| Time=2009 | 0† | |||
| Condition=Pull | 0.682±1.030 | 0.508 | ||
| Condition=Push | −3.218±1.293 | 0.013 | ||
| Condition=Free | 0† | |||
| Condition=Pull*Time=2007 | 0.372±0.380 | 0.328 | ||
| Condition=Push*Time=2007 | −2.470±0.783 | 0.002 | ||
| Condition=Free*Time=2007 | 0† | |||
| Condition=Pull*Time=2009 | 0† | |||
| Condition=Push*Time=2009 | 0† | |||
| Condition=Free*Time=2009 | 0† |
Best-fitting model assessed with a binomial generalized linear mixed model (GLMM) with a logit link function. Group and Individual identity were entered as random factors. Test statistics (significant results in bold) and coefficients ± SE of significant results are also shown. Reference category: pull.
This parameter is set to zero because it is redundant.
Table A4.
Comparison of experienced and freshly trained individuals (first test block) of the pull and push condition
| Variable | F | df | Coefficient±SE | P |
|---|---|---|---|---|
| Condition | 19.544 | 1 | <0.001 | |
| Condition=Pull | 6.084±1.376 | <0.001 | ||
| Condition=Push | 0† |
Best-fitting model assessed with a binomial generalized linear mixed model (GLMM) with a logit link function. Group and Individual identity were entered as random factors. Test statistics (significant result in bold) and coefficients ± SE of significant results are shown. Reference category: push.
This parameter is set to zero because it is redundant.
Table A5.
Naïve individuals
| Variable | F | df | Coefficient±SE | P |
|---|---|---|---|---|
| All naïve individuals included (‘present’ and ‘not present’) | ||||
| Age | 6.975 | 1 | 0.008 | |
| Condition*Presence | 6.198 | 6 | <0.001 | |
| Age=Juveniles | 1.476±0.559 | 0.008 | ||
| Age=Adults | 0† | |||
| Condition=Pull*Presence=yes | −2.132±0.715 | 0.003 | ||
| Condition=Pull*Presence=no | −0.811±2.275 | 0.721 | ||
| Condition=Push*Presence=yes | 1.342±0.546 | 0.014 | ||
| Condition=Push*Presence=no | −2.914±1.084 | 0.007 | ||
| Condition=Free Pull*Presence=yes | −2.952±0.841 | <0.001 | ||
| Condition=Free Pull*Presence=no | −1.576±2.233 | 0.480 | ||
| Condition=Free Push*Presence=yes | 0† | |||
| Only ‘present’ naïve individuals included | ||||
| Condition | 14.394 | 3 | <0.001 | |
| Age | 6.759 | 1 | 0.009 | |
| Condition*Age | 1.852 | 3 | 0.135 | |
| Condition=Pull | −1.243±0.708 | 0.079 | ||
| Condition=Push | 2.712±0.584 | <0.001 | ||
| Condition=Free Pull | −1.202±0.837 | 0.151 | ||
| Condition=Free Push | 0† | |||
| Age=Juveniles | 0.318±0.814 | 0.696 | ||
| Age=Adults | 0† |
Best-fitting model assessed with a binomial generalized linear mixed model (GLMM) with a logit link function. Group and Individual identity were entered as random factors. Test-statistics (significant results in bold) and coefficients ± SE of significant results are also shown. Reference category: pull.
This parameter is set to zero because it is redundant.
Table A6.
Comparison of naïve and experienced individuals
| Variable | F | df | Coefficient±SE | P |
|---|---|---|---|---|
| Condition | 14.318 | 3 | <0.001 | |
| Age | 8.370 | 1 | 0.004 | |
| Condition*Age | 2.706 | 3 | 0.044 | |
| Condition*Sex | 1.492 | 4 | 0.202 | |
| Condition=Pull | −2.568±0.932 | 0.006 | ||
| Condition=Push | 2.840±0.734 | <0.001 | ||
| Condition=Free Pull | −1.268±0.975 | 0.193 | ||
| Condition=Free Push | 1.162† | |||
| Age=Juvenile | 0.531±1.147 | 0.643 | ||
| Age=Adult | 0† | |||
| Condition=Pull*Age=Juvenile | 2.839±1.359 | 0.037 | ||
| Condition=Pull*Age=Adult | 0† | |||
| Condition=Push*Age=Juvenile | −0.342±1.496 | 0.819 | ||
| Condition=Push*Age=Adult | 0† | |||
| Condition=Free Pull*Age=Juvenile | 1.304±1.507 | 0.387 | ||
| Condition=Free Pull*Age=Adult | 0† | |||
| Condition=Free Push*Age=Juvenile | 0† | |||
| Condition=Free Push*Age=Adult | 0† |
Best-fitting model assessed with a binomial generalized linear mixed model (GLMM) with a logit link function. Group and Individual identity were entered as random factors. Test-statistics (significant results in bold) and coefficients ± SE of significant results are also shown. Reference category: pull.
This parameter is set to zero because it is redundant.
Table A7.
Successful manipulations
| Variable | F | df | Coefficient±SE | P |
|---|---|---|---|---|
| All individuals are included | ||||
| Condition | 1.171 | 3 | 0.319 | |
| Age | 16.070 | 1 | <0.001 | |
| Condition*Age | 0.781 | 3 | 0.504 | |
| Condition*Presence | 8.505 | 3 | <0.001 | |
| Age=Juvenile | 2.796±1.589 | 0.079 | ||
| Age=Adult | 0† | |||
| Condition=Pull*Presence=no | 3.304±2.516 | 0.189 | ||
| Condition=Pull*Presence=yes | 0† | |||
| Condition=Push*Presence=no | −5.099±1.068 | <0.001 | ||
| Condition=Push*Presence=yes | 0† | |||
| Condition=Free Pull*Presence=no | 2.335±2.471 | 0.345 | ||
| Condition=Free Pull*Presence=yes | 0† | |||
| Condition=Free Push*Presence=yes | 0† | |||
| Only ‘present’ individuals are included | ||||
| Condition | 19.808 | 3 | <0.001 | |
| Age | 23.264 | 1 | <0.001 | |
| Condition=Pull | −3.665±0.807 | <0.001 | ||
| Condition=Push | 1.965±0.799 | 0.014 | ||
| Condition=Free Pull | −3.745±0.932 | <0.001 | ||
| Condition=Free Push | 0† | |||
| Age=Juvenile | 2.978±0.617 | <0.001 | ||
| Age=Adult | 0† |
Best-fitting model assessed with a binomial generalized linear mixed model (GLMM) with a logit link function. Group and Individual identity were entered as random factors. Test statistics (significant results in bold) and coefficients ± SE of significant results are also shown. Reference category: pull.
This parameter is set to zero because it is redundant.
Table A8.
Efficiency
| Variable | F | df | Coefficient±SE | P |
|---|---|---|---|---|
| Block | 21.635 | 2 | <0.001 | |
| Age | 27.625 | 1 | <0.001 | |
| Technique | 1632.001 | 1 | <0.001 | |
| Block*Technique | 39.444 | 2 | <0.001 | |
| Sex*Technique | 0.637 | 1 | 0.425 | |
| Sex | 0.126 | 1 | 0.722 | |
| Block=1 | 0.900±0.098 | <0.001 | ||
| Block=2 | 0.227±0.098 | 0.021 | ||
| Block=3 | 0† | |||
| Age=Juvenile | 0.942±0.179 | <0.001 | ||
| Age=Adult | 0† | |||
| Technique=Push | 3.481±0.122 | <0.001 | ||
| Technique=Pull | 0† | |||
| Block=1*Technique=Pull | −1.004±0.121 | <0.001 | ||
| Block=1*Technique=Push | 0† | |||
| Block=2*Technique=Pull | −0.196±0.121 | 0.105 | ||
| Block=2*Technique=Push | 0† | |||
| Block=3*Technique=Pull | 0† | |||
| Block=3*Technique=Push | 0† |
Best-fitting model assessed with a binomial generalized linear mixed model (GLMM) with a logit link function. Group and Individual identity were entered as random factors. Test statistics (significant results in bold) and coefficients ± SE of significant results are also shown. Reference category: success.
This parameter is set to zero because it is redundant.
Table A9.
Persistence
| Variable | F | df | Coefficient±SE | P |
|---|---|---|---|---|
| Condition | 12.505 | 3 | <0.001 | |
| Session number | 187.220 | 1 | <0.001 | |
| Sex | 0.002 | 1 | 0.967 | |
| Age | 13.796 | 1 | <0.001 | |
| Condition*Session number | 11.174 | 3 | <0.001 | |
| Condition*Sex | 2.051 | 3 | 0.104 | |
| Condition*Age | 3.166 | 3 | 0.023 | |
| Condition=Pull | −0.630±0.627 | 0.315 | ||
| Condition=Push | 0.817±0.485 | 0.092 | ||
| Condition=Free Pull | −2.005±0.629 | 0.001 | ||
| Condition=Free Push | 0† | |||
| Session number | 0.086±0.009 | <0.001 | ||
| Age=Juvenile | −0.093±0.791 | 0.907 | ||
| Age=Adult | 0† | |||
| Condition=Pull*Session number | −0.062±0.011 | <0.001 | ||
| Condition=Push*Session number | −0.044±0.011 | <0.001 | ||
| Condition=Free Pull*Session number | −0.047±0.011 | <0.001 | ||
| Condition=Free Push*Session number | 0† | |||
| Condition=Pull*Age=Juvenile | 2.716±0.935 | 0.004 | ||
| Condition=Pull*Age=Adult | 0† | |||
| Condition=Push*Age=Juvenile | 1.110±0.974 | 0.254 | ||
| Condition=Push*Age=Adult | 0† | |||
| Condition=Free Pull*Age=Juvenile | 1.566±1.034 | 0.130 | ||
| Condition=Free Pull*Age=Adult | 0† | |||
| Condition=Free Push*Age=Juvenile | 0† | |||
| Condition=Free Push*Age=Adult | 0† |
Best-fitting model assessed with a binomial generalized linear mixed model (GLMM) with a logit link function. Group and Individual identity were entered as random factors. Test statistics (significant results in bold) and coefficients ± SE of significant results are also shown. Reference category: pull.
This parameter is set to zero because it is redundant.
References
- Acerbi A., Jacquet P.O.P., Tennie C. Behavioral constraints and the evolution of faithful social learning. Current Zoology. 2012;58(2):307–318. [Google Scholar]
- Allen J., Weinrich M., Hoppitt W., Rendell L. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science. 2013;340(6131):485–488. doi: 10.1126/science.1231976. [DOI] [PubMed] [Google Scholar]
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–266. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Biro D., Inoue-Nakamura N., Tonooka R., Yamakoshi G., Sousa C., Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Animal Cognition. 2003;6(4):213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Bonnie K.E., Horner V., Whiten A., de Waal F.B.M. Spread of arbitrary conventions among chimpanzees: a controlled experiment. Proceedings of the Royal Society B: Biological Sciences. 2007;274(1608):367–372. doi: 10.1098/rspb.2006.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box H., Yamamoto M., Lopes F. Gender differences in marmosets and tamarins: responses to food tasks. International Journal of Comparative Psychology. 1999;12(2):59–70. [Google Scholar]
- Boyd R., Richerson P.J. University of Chicago Press; Chicago, IL: 1985. Culture and the evolutionary process. [Google Scholar]
- Boyd R., Richerson P.J. Oxford University Press; Oxford, UK: 2005. The origin and evolution of cultures. [Google Scholar]
- Bugnyar T., Huber L. Push or pull: an experimental study on imitation in marmosets. Animal Behaviour. 1997;54(4):817–831. doi: 10.1006/anbe.1996.0497. [DOI] [PubMed] [Google Scholar]
- Burkart J.M., Fehr E., Efferson C., van Schaik C.P. Other-regarding preferences in a non-human primate: common marmosets provision food altruistically. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):19762–19766. doi: 10.1073/pnas.0710310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell C.A., Whiten A. Scrounging facilitates social learning in common marmosets, Callithrix jacchus. Animal Behaviour. 2003;65(6):1085–1092. [Google Scholar]
- Campbell F., Heyes C., Goldsmith A. Stimulus learning and response learning by observation in the European starling, in a two-object/two-action test. Animal Behaviour. 1999;58(1):151–158. doi: 10.1006/anbe.1999.1121. [DOI] [PubMed] [Google Scholar]
- Claidière N., Bowler M., Whiten A. Evidence for weak or linear conformity but not for hyper-conformity in an everyday social learning context. PLoS One. 2012;7(2):e30970. doi: 10.1371/journal.pone.0030970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claidière N., Sperber D. Imitation explains the propagation, not the stability of animal culture. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1681):651–659. doi: 10.1098/rspb.2009.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claidière N., Whiten A. Integrating the study of conformity and culture in humans and nonhuman animals. Psychological Bulletin. 2012;138(1):126–145. doi: 10.1037/a0025868. [DOI] [PubMed] [Google Scholar]
- Coussi-Korbel S., Fragaszy D.M. On the relation between social dynamics and social learning. Animal Behaviour. 1995;50(6):1441–1453. [Google Scholar]
- Crast J., Hardy J.M., Fragaszy D.M. Inducing traditions in captive capuchin monkeys (Cebus apella) Animal Behaviour. 2010;80(6):955–964. [Google Scholar]
- Digby L.J. Social organization in a wild population of Callithrix jacchus: II. Intragroup social behavior. Primates. 1995;36(3):361–375. [Google Scholar]
- Dindo M., Whiten A., de Waal F.B.M. In-group conformity sustains different foraging traditions in capuchin monkeys (Cebus apella) PLoS One. 2009;4(11):e7858. doi: 10.1371/journal.pone.0007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferson C., Lalive R., Richerson P.J., McElreath R., Lubell M. Conformists and mavericks: the empirics of frequency-dependent cultural transmission. Evolution and Human Behavior. 2008;29(1):56–64. [Google Scholar]
- Fragaszy D.M., Perry S. Towards a biology of traditions. In: Fragaszy D.M., Perry S., editors. The biology of traditions: Models and evidence. Cambridge University Press; Cambridge, UK: 2003. pp. 1–32. [Google Scholar]
- Fragaszy D., Visalberghi E. Socially biased learning in monkeys. Learning & Behavior. 2004;32(1):24–35. doi: 10.3758/bf03196004. [DOI] [PubMed] [Google Scholar]
- Franz M., Matthews L.J. Social enhancement can create adaptive, arbitrary and maladaptive cultural traditions. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1698):3363–3372. doi: 10.1098/rspb.2010.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz M., Nunn C.L. Network-based diffusion analysis: a new method for detecting social learning. Proceedings of the Royal Society B: Biological Sciences. 2009;276(1663):1829–1836. doi: 10.1098/rspb.2008.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef B.G., Jr. Social transmission of acquired behavior: a discussion of tradition and social learning in vertebrates. Advances in the Study of Behavior. 1976;3:77–100. [Google Scholar]
- Galef B.G., Jr. Approaches to the study of traditional behaviors of free-living animals. Learning & Behavior. 2004;32(1):53–61. doi: 10.3758/bf03196006. [DOI] [PubMed] [Google Scholar]
- Galef B.G., Jr., Manzig L.A., Field R.M. Imitation learning in budgerigars: Dawson and Foss (1965) revisited. Behavioural Processes. 1986;13(1–2):191–202. doi: 10.1016/0376-6357(86)90025-2. [DOI] [PubMed] [Google Scholar]
- Gunhold, T. Massen, J. J. M. M., Schiel, N., Souto A, & Bugnyar, T. (n.d.). Proximity and tolerance between wild common marmosets in an artificial fruit task. Unpublished raw data.
- Heyes C. Social learning in animals: categories and mechanisms. Biological Reviews. 1994;69(2):207–231. doi: 10.1111/j.1469-185x.1994.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Heyes C., Dawson G. A demonstration of observational learning in rats using a bidirectional control. The Quarterly Journal of Experimental Psychology Section B. 1990;42:59–71. [PubMed] [Google Scholar]
- Hopper L.M., Schapiro S.J., Lambeth S.P., Brosnan S.F. Chimpanzees’ socially maintained food preferences indicate both conservatism and conformity. Animal Behaviour. 2011;81(6):1195–1202. doi: 10.1016/j.anbehav.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper L.M., Spiteri A., Lambeth S.P., Schapiro S.J., Horner V., Whiten A. Experimental studies of traditions and underlying transmission processes in chimpanzees. Animal Behaviour. 2007;73(6):1021–1032. [Google Scholar]
- Hoppitt W.J., Laland K.N. Detecting social learning using networks: a users guide. American Journal of Primatology. 2011;73(8):834–844. doi: 10.1002/ajp.20920. [DOI] [PubMed] [Google Scholar]
- Hoppitt W., Kandler A., Kendal J.R., Laland K.N. The effect of task structure on diffusion dynamics: implications for diffusion curve and network-based analyses. Learning & Behavior. 2010;38(3):243–251. doi: 10.3758/LB.38.3.243. [DOI] [PubMed] [Google Scholar]
- Horner V., Proctor D., Bonnie K.E., Whiten A., de Waal F.B.M. Prestige affects cultural learning in chimpanzees. PLoS One. 2010;5(5):e10625. doi: 10.1371/journal.pone.0010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrubesch C., Preuschoft S., van Schaik C. Skill mastery inhibits adoption of observed alternative solutions among chimpanzees (Pan troglodytes) Animal Cognition. 2009;12(2):209–216. doi: 10.1007/s10071-008-0183-y. [DOI] [PubMed] [Google Scholar]
- Huffman M.M.A., Hirata S. Biological and ecological foundations of primate behavioral tradition. In: Fragaszy D.M., Perry S., editors. The Biology of traditions: Models and evidence. Cambridge University Press; Cambridge, UK: 2003. pp. 267–296. [Google Scholar]
- Kendal R.L., Galef B.G., Jr., van Schaik C.P. Social learning research outside the laboratory: how and why? Learning & Behavior. 2010;38(3):187–194. doi: 10.3758/LB.38.3.187. [DOI] [PubMed] [Google Scholar]
- Kendal R.L., Kendal J.R., Hoppitt W., Laland K.N. Identifying social learning in animal populations: a new ‘option-bias’ method. PLoS One. 2009;4(8):e6541. doi: 10.1371/journal.pone.0006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal R.L., Custance D.D.M., Kendal J.J.R., Vale G., Stoinski T.S., Rakotomalala N.L. Evidence for social learning in wild lemurs (Lemur catta) Learning & Behavior. 2010;38(3):220–234. doi: 10.3758/LB.38.3.220. [DOI] [PubMed] [Google Scholar]
- Lachlan R.F., Janik V.M., Slater P.J.B. The evolution of conformity-enforcing behaviour in cultural communication systems. Animal Behaviour. 2004;68(3):561–570. [Google Scholar]
- Laland K.N. Social learning strategies. Learning & Behavior. 2004;32(1):4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- Laland K.N., Janik V.M. The animal cultures debate. Trends in Ecology & Evolution. 2006;21(10):542–547. doi: 10.1016/j.tree.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Laland K.N., Odling-Smee J., Feldman M.W. Niche construction, biological evolution, and cultural change. Behavioral and Brain Sciences. 2000;23(1):131–146. doi: 10.1017/s0140525x00002417. [DOI] [PubMed] [Google Scholar]
- Leca J.-B., Huffman M., Gunst N. Japanese macaque cultures: inter- and intra-troop behavioural variability of stone handling patterns across 10 troops. Behaviour. 2007;144(3):251–281. [Google Scholar]
- Lehner P. Cambridge University Press; Cambridge, UK: 1996. Handbook of ethological methods. [Google Scholar]
- Lindeyer C.M., Reader S.M. Social learning of escape routes in zebrafish and the stability of behavioural traditions. Animal Behaviour. 2010;79(4):827–834. [Google Scholar]
- Marshall-Pescini S., Whiten A. Chimpanzees (Pan troglodytes) and the question of cumulative culture: an experimental approach. Animal Cognition. 2008;11(3):449–456. doi: 10.1007/s10071-007-0135-y. [DOI] [PubMed] [Google Scholar]
- Miller H.C., Rayburn-Reeves R., Zentall T.R. Imitation and emulation by dogs using a bidirectional control procedure. Behavioural Processes. 2009;80(2):109–114. doi: 10.1016/j.beproc.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C.A., Cant M.A. Imitation and traditions in wild banded mongooses. Current Biology. 2010;20(13):1171–1175. doi: 10.1016/j.cub.2010.04.037. [DOI] [PubMed] [Google Scholar]
- Nicol C.J., Pope S.J. The effects of demonstrator social status and prior foraging success on social learning in laying hens. Animal Behaviour. 1999;57(1):163–171. doi: 10.1006/anbe.1998.0920. [DOI] [PubMed] [Google Scholar]
- Ottoni E.B., Izar P. Capuchin monkey tool use: overview and implications. Evolutionary Anthropology: Issues, News, and Reviews. 2008;17(4):171–178. [Google Scholar]
- Perry S., Baker M., Fedigan L., Gros-Louis J., Jack K., MacKinnon K.C. Social conventions in wild white-faced capuchin monkeys: evidence for traditions in a neotropical primate. Current Anthropology. 2003;44(2):241–268. [Google Scholar]
- Pesendorfer M.B., Gunhold T., Schiel N., Souto A., Huber L., Range F. The maintenance of traditions in marmosets: individual habit, not social conformity? A field experiment. PLoS One. 2009;4(2):e4472. doi: 10.1371/journal.pone.0004472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price E.E., Whiten A. Social learning in primates. In: Wasserman E.A., Zentall T.R., editors. The Oxford Handbook of Comparative Cognition. Oxford University Press; New York: 2012. pp. 862–880. [Google Scholar]
- Reader S.M., Biro D. Experimental identification of social learning in wild animals. Learning & Behavior. 2010;38(3):265–283. doi: 10.3758/LB.38.3.265. [DOI] [PubMed] [Google Scholar]
- Rendell L., Whitehead H. Culture in whales and dolphins. Behavioral and Brain Sciences. 2001;24(02):309–324. doi: 10.1017/s0140525x0100396x. [DOI] [PubMed] [Google Scholar]
- van Schaik C.P., Ancrenaz M., Borgen G., Galdikas B., Knott C.D., Singleton I. Orangutan cultures and the evolution of material culture. Science. 2003;299(5603):102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- Schiel N., Huber L. Social influences on the development of foraging behavior in free-living common marmosets (Callithrix jacchus) American Journal of Primatology. 2006;68(12):1150–1160. doi: 10.1002/ajp.20284. [DOI] [PubMed] [Google Scholar]
- Schiel N., Souto A., Bezerra B.M., Huber L. A stress-free method of identifying common marmosets (Callithrix jacchus) in the wild. In: Ferrari S., Rimoli J., editors. Vol. 9. Sociedade Brasileira de Primatologia; Aracaju: 2008. pp. 147–153. (A primatologia no Brasil). [Google Scholar]
- Schnoell A.V., Fichtel C. Wild redfronted lemurs (Eulemur rufifrons) use social information to learn new foraging techniques. Animal Cognition. 2012;15(4):505–516. doi: 10.1007/s10071-012-0477-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto A., Bezerra B.M., Schiel N., Huber L. Saltatory search in free-living Callithrix jacchus: environmental and age influences. International Journal of Primatology. 2007;28(4):881–893. [Google Scholar]
- Tardif S., Harrison M., Simek M. Communal infant care in marmosets and tamarins: relation to energetics, ecology, and social organization. In: Rylands A.B., editor. Marmosets and tamarins: Systematics, behaviour and ecology. Oxford University Press; Oxford, UK: 1993. pp. 220–234. [Google Scholar]
- Tennie C., Call J., Tomasello M. Push or pull: imitation vs. emulation in great apes and human children. Ethology. 2006;112(12):1159–1169. [Google Scholar]
- Thornton A., Clutton-Brock T. Social learning and the development of individual and group behaviour in mammal societies. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366(1567):978–987. doi: 10.1098/rstb.2010.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A., Malapert A. Experimental evidence for social transmission of food acquisition techniques in wild meerkats. Animal Behaviour. 2009;78(2):255–264. [Google Scholar]
- Thornton A., Malapert A. The rise and fall of an arbitrary tradition: an experiment with wild meerkats. Proceedings of the Royal Society B: Biological Sciences. 2009;276(1660):1269–1276. doi: 10.1098/rspb.2008.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A., Samson J., Clutton-Brock T. Multi-generational persistence of traditions in neighbouring meerkat groups. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1700):3623–3629. doi: 10.1098/rspb.2010.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M., Carpenter M., Call J., Behne T., Moll H. Understanding and sharing intentions: the origins of cultural cognition. The Behavioral and Brain Sciences. 2005;28(5):675–735. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Voelkl B., Huber L. True imitation in marmosets. Animal Behaviour. 2000;60(2):195–202. doi: 10.1006/anbe.2000.1457. [DOI] [PubMed] [Google Scholar]
- van de Waal E., Borgeaud C., Whiten A. Potent social learning and conformity shape a wild primate's foraging decisions. Science. 2013;340(6131):483–485. doi: 10.1126/science.1232769. [DOI] [PubMed] [Google Scholar]
- van de Waal E., Bshary R. Social-learning abilities of wild vervet monkeys in a two-step task artificial fruit experiment. Animal Behaviour. 2011;81(2):433–438. [Google Scholar]
- van de Waal E., Bshary R., Whiten A. Wild vervet monkey infants acquire the food-processing variants of their mothers. Animal Behaviour. 2014;90:41–45. [Google Scholar]
- van de Waal E., Claidière N., Whiten A. Social learning and spread of alternative means of opening an artificial fruit in four groups of vervet monkeys. Animal Behaviour. 2013;85(1):71–76. [Google Scholar]
- van de Waal E., Renevey N., Favre C.M., Bshary R. Selective attention to philopatric models causes directed social learning in wild vervet monkeys. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1691):2105–2111. doi: 10.1098/rspb.2009.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A. The second inheritance system of chimpanzees and humans. Nature. 2005;437(7055):52–55. doi: 10.1038/nature04023. [DOI] [PubMed] [Google Scholar]
- Whiten A., Goodall J., McGrew W.C., Nishida T., Reynolds V., Sugiyama Y. Cultures in chimpanzees. Nature. 1999;399(6737):682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Whiten A., Horner V., de Waal F.B.M. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437(7059):737–740. doi: 10.1038/nature04047. [DOI] [PubMed] [Google Scholar]
- Whiten A., Mesoudi A. Establishing an experimental science of culture: animal social diffusion experiments. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1509):3477–3488. doi: 10.1098/rstb.2008.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A., Spiteri A., Horner V., Bonnie K.E., Lambeth S.P., Schapiro S.J. Transmission of multiple traditions within and between chimpanzee groups. Current Biology. 2007;17(12):1038–1043. doi: 10.1016/j.cub.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Zentall T.R. Imitation: definitions, evidence, and mechanisms. Animal Cognition. 2006;9(4):335–353. doi: 10.1007/s10071-006-0039-2. [DOI] [PubMed] [Google Scholar]