Abstract
The etiologic role of genetic and environmental factors on disordered eating was examined in a sample of 15- to 17-year-old female–female, male–male, and opposite-sex twin pairs. Also assessed was whether a single factor is underlying 3 facets (body dissatisfaction, drive for thinness, bulimia) of disordered eating, including the possible importance of sex differences. Univariate model-fitting analyses indicated that genetic factors are more important for girls and environment more important for boys for body dissatisfaction and drive for thinness. A multivariate common factor analysis indicated that a single factor accounted for the association among these 3 facets of disordered eating in both sexes. However, only 50% of the genetic risk for this factor is shared between the sexes.
Keywords: eating disorder, disordered eating, twin study, genetics, sex differences
Eating disorders (EDs) occur in approximately 0.5–3.0% of the population, with more affected females than males (American Psychiatric Association [APA], 2000). The female-to-male ratio of ED diagnoses in nonclinical populations has been estimated at 10:1 (APA, 2000). However, recent research indicates a ratio of 4:1 for anorexia nervosa (AN; Woodside et al., 2001). Even though a convergence in ED prevalence is being seen between the sexes, a sex difference still exists. Environmental and genetic factors, including sociocultural pressures to be thin in females (Andersen & Holman, 1997; Ricciardelli & McCabe, 2004; Stice, 1994) and reproductive hormones (Edler, Lipson, & Keel, 2006; Klump et al., 2005; Klump, Keel, Culbert, & Edler, 2008), have been invoked to account for this. However, whether fewer males develop an ED because they are exposed to fewer societal pressures to be thin or because they have a biological protection against an ED continues to be a topic of debate (Andersen, 1999).
Sex Differences in EDs
Until recently, research examining EDs in males and sex differences in EDs focused on individual cases or small clinical samples. This research revealed the clinical presentation and course, symptomatology, medical complications, and prognosis of EDs are comparable between the sexes (Bosch-Bramon, Troop, & Treasure, 2000; Carlat, Camargo, & Herzog, 1997; Crisp, Burns, & Bhat, 1986; Geist, Heinmaa, Katzman, & Stephens, 1999; Woodside et al., 2001). Currently, research has shifted to focus on larger community-based samples of males to examine etiologic factors. These studies revealed similarities across sexes in the risk for EDs and disordered eating (Croll, Neumark-Sztainer, Story, & Ireland, 2002; Muise, Stein, & Arbess, 2003; Neumark-Sztainer & Hannan, 2000; Olivardia, Pope, Mangweth, & Hudson, 1995; Woodside et al., 2001). For example, body mass index, negative affect, societal pressure to be thin, and body dissatisfaction are associated with the development of disordered eating and EDs in both females (see Jacobi, Hayward, de Zwaan, Kraemer, & Agras, 2004, for a review) and males (see Ricciardelli & McCabe, 2004, for a review). It is unclear, however, whether body dissatisfaction is a risk for an ED or a prodromal symptom.
Finally, some important differences have been noted. First, the manifestation of body dissatisfaction is different between the sexes. Males tend to be more dissatisfied with their bodies when they are underweight or overweight, whereas females are most satisfied when underweight (Cohane & Pope, 2001; Presnell, Bearman, & Stice, 2004; Ricciardelli & McCabe, 2001). Second, homosexuality appears to be exclusively associated with ED development in males, whereas a masculine homosexual orientation in females is a possible protective factor (e.g., Meyer, Blissett, & Oldfield, 2001; Russell & Keel, 2002). Finally, males with an ED demonstrate increased rates of excessive exercise and a lower prevalence of dieting as compensatory behaviors and more frequently exhibit premorbid obesity (e.g., Anderson & Bulik, 2004; Lewinsohn, Seeley, Moerk, & Striegel-Moore, 2002; Ricciardelli & McCabe, 2004).
Genetic Risk for EDs
Multiple studies have been conducted to delineate the genetic epidemiology of EDs. Twin studies report heritability estimates for AN in females to vary between 22% and 76% (Bulik et al., 2006; Klump, Miller, Keel, McGue, & Iacono, 2001; Mazzeo et al., 2008; Wade, Bulik, & Kendler, 2000), whereas estimates for bulimia nervosa (BN) vary between 54% and 83% (Bulik, Sullivan, & Kendler, 1998; Bulik, Sullivan, Wade, & Kendler, 2000; Kendler et al., 1991, 1995). According to these same studies, unique environment (which reflects those experiences that make twins dissimilar) is more important than shared environment (which results from environmental experiences that make twins similar; Kendler & Prescott, 2006). However, given the wide estimates for confidence intervals for shared environment in these studies, we cannot eliminate it definitively as an influence (Bulik et al., 2000).
Two studies also suggest a common latent factor is responsible for the genetic influences on disordered eating in females, specifically for aspects of disinhibition (i.e., disinhibition of control of eating in response to a variety of cues) and hunger (B. M. Neale, Mazzeo, & Bulik, 2003). The covariation between three assessments of disordered eating (i.e., self-report, semi-structured psychiatric interview, semi-structured ED interview) was examined, and a single latent factor was also found to explain covariance (Wade et al., 1999). However, it is still unknown if an underlying factor is responsible for other aspects of disordered eating and if this same structure is found in males.
Extensive research has attempted to tease apart the contribution of psychological and social factors to sex differences in ED risk. An alternate approach is to search for the existence and nature of sex differences in genetic risk. Using this approach, we are able to assess whether the genetic factors influencing the development of an ED in males and females are the same or at least partially distinct.
We are aware of eight studies examining the heritability of disordered eating in males, seven of which include a female comparison group (Eiben, 2007; Keski-Rahkonen, Bulik, et al., 2005; Keski-Rahkonen, Neale, et al., 2005; Reichborn-Kjennerud et al., 2003; Reichborn-Kjennerud, Bulik, Kendler, et al., 2004; Reichborn-Kjennerud, Bulik, Tambs, & Harris, 2004; Slane, Burt, & Klump, 2007; Tholin, Rasmussen, Tynelius, & Karlsson, 2005). Although the results of these studies are somewhat discrepant, the most consistent finding is lower heritability estimates for males compared with females.
For example, two studies examined aspects of body dissatisfaction and drive for thinness and showed lower heritability estimates for males than for females (Eiben, 2007; Keski-Rahkonen, Bulik, et al., 2005). However, results are discrepant between these studies with regard to environmental factors. Results revealed by Eiben (2007) suggested genetic and shared environmental factors are important for males, whereas results by Keski-Rahkonen, Bulik, et al. (2005) suggested no genetic influence, only shared and unique environmental factors. Specifically, Eiben reported heritability estimates for body dissatisfaction at 37% and 58% and for drive for thinness at 23% and 41% in males and females, respectively. Lower heritabilities have also been exhibited for a measure of intentional weight loss in males (38% for males vs. 66% for females). However, no shared environmental factors were indicated for males (Keski-Rahkonen, Neale, et al., 2005). Finally, in examining aspects of cognitive restraint, emotional eating, and uncontrolled eating, Tholin et al. (2005) found that heritability estimates ranged from 45– 60% in males, whereas shared environment was not shown to contribute to eating behavior. However, there was no female comparison group.
Inconsistencies could be because of two main factors. First, only two of the aforementioned studies used the same assessment instrument (Eiben, 2007; Keski-Rahkonen, Bulik, et al., 2005), making comparisons across studies difficult. However, even these two studies show discrepant results. This may be because the authors scored their measures of disordered eating differently. The age of the participants in these samples also varies, ranging from adolescence to adulthood.
The abovementioned studies also have one major limitation: They fit twin models to male and female data separately and compared parameter estimates by inspection. This approach does not permit for a rigorous examination of quantitative or qualitative sex differences because it does not use sex-limitation twin models. Quantitative effects answer the question as to whether genetic factors are more important for the etiology of disordered eating in males or females (Kendler & Prescott, 2006). Although it is possible to fit quantitative models with only same-sex twin pairs, opposite-sex twin pairs are needed to examine for qualitative effects. Qualitative effects examine whether the same genes are involved in the etiology of disordered eating in the sexes (Kendler & Prescott, 2006).
Four studies have examined quantitative or qualitative sex effects (Reichborn-Kjennerud et al., 2003; Reichborn-Kjennerud, Bulik, Kendler, et al., 2004; Reichborn-Kjennerud, Bulik, Tambs, & Harris, 2004; Slane et al., 2007). Two studies by Reichborn-Kjennerud et al. (2003; Reichborn-Kjennerud, Bulik, Kendler, et al., 2004) examined quantitative and qualitative sex effects on binge eating. The first examination showed no quantitative effects (i.e., the magnitudes of the genetic and environmental effects were the same in male and females). However, results indicated qualitative effects (Reichborn-Kjennerud et al., 2003). This suggests the genetic factors influencing binge eating are not entirely the same in both sexes. In the follow-up examination, however, excluding those individuals who reported compensatory behaviors, no quantitative or qualitative sex differences were exhibited (Reichborn-Kjennerud, Bulik, Tambs, & Harris, 2004). Similarly, Slane et al. (2007) reported no quantitative sex differences on the facets of weight preoccupation and binge eating. Quantitative effects were, however, indicated for body dissatisfaction, with females having a higher heritability than males (Slane et al., 2007). Finally, one additional study revealed no quantitative or qualitative sex effects on a measure of the influence of weight on self-evaluation (Reichborn-Kjennerud, Bulik, Kendler, et al., 2004).
Taken together, results appear somewhat inconsistent. Although most of the previous studies indicate lower heritability estimates for males compared with females, results in regard to environmental influences are discrepant. Some studies suggest that both genetic and shared environmental factors are important for males, whereas others indicate only environmental factors are of importance. In sum, however, results from the studies reviewed suggest that environmental factors are more important for aspects of body dissatisfaction and drive for thinness in males than in females. Studies also suggest similar heritability estimates in males and females for binge eating.
The Current Study
For the present study, we used an epidemiological sample of 15-to 17-year-old Swedish male and female same-sex twin pairs and opposite-sex twin pairs. Our purpose is to answer the following questions: (a) Are there quantitative and qualitative sex differences in the genetic risk factors for aspects of body dissatisfaction, drive for thinness, and bulimia; (b) is there a single latent factor underlying these three facets of disordered eating; and (c) are there sex differences in the genetic and environmental risk factors on this latent factor?
This study improves on the limitations of previous studies in several ways. First, we are using an adolescent sample. Adolescence is consistently shown to be the greatest period of risk for disordered eating, especially in females (APA, 2000; Hudson, Hiripi, Pope, & Kessler, 2007). Our population also has a much higher response rate than most of the previous studies discussed. Third, we are examining the structure of disordered eating (i.e., whether a single underlying factor is responsible). Finally, we are fitting a complete sex-limitation model.
Method
Participants
The present sample, the Swedish Twin Study of Child and Adolescent Development (TCHAD), began with all twin pairs born in Sweden between May 1985 and December 1986 (Lichtenstein, Tuvblad, Larsson, & Carlström, 2007). The sample includes 246 and 238 monozygotic (MZ) and 181 and 169 dizygotic (DZ) female–female and male–male twin pairs, respectively, and 366 opposite-sex twin pairs. Twin pairs were recruited through the Medical Birth Registry: Identified twins and their parents were mailed study questionnaires (Lichtenstein & Svartengren, 1997). For Wave 1 assessments, only parents of twins were recruited. In Wave 2, twin self-reports were also assessed and 78% of the twins responded. For the present study, information from Wave 3, when the twins were 15 to 17 years old, are used. The response rate at Wave 3 for all twins contacted was 82% (Lichtenstein et al., 2007). Ninety-two percent of twins who responded at Wave 2 responded to Wave 3 questionnaires (Lichtenstein et al., 2007).
Analyses have been conducted to investigate the effect of attrition on the data. Results show nonsignificant results for those subjects lost to follow-up at Wave 3 and responders on twin self-reports of antisocial behavior and sex ratio (Lichtenstein et al., 2007). Similarly, no significant differences were found for parental self-reports of unemployment level, educational level, buying power, or neighborhood crime rate. However, nonparticipating families did tend to live in more ethnically diverse neighborhoods, and, when we used parent-report data, we found that those twins lost to follow-up at Wave 3 also showed a significantly elevated risk for hyperactivity–impulsivity (Lichtenstein et al., 2007).
The questionnaires used in the TCHAD were approved by the Ethics Committee of the Karolinska Institute, Stockholm, Sweden. Informed consent is not required, as responding to the questionnaire constitutes consent in Sweden.
Zygosity Determination
Zygosity of twin pairs is determined on the basis of computer algorithms of questionnaire responses (Lichtenstein et al., 2007). These questions were validated by a discriminant analysis of 106 same-sex pairs where zygosity had been determined by typing 16 polymorphic DNA markers (Lichtenstein et al., 2007).
Measures
Symptoms of disordered eating were examined with the Eating Disorder Inventory–II (EDI; Garner, 1991). This Swedish version of the EDI has been translated and validated on a female population (Nevonen, Clinton, & Norring, 2006; Norring & Sohlberg, 1988). The Drive for Thinness (i.e., excessive concern with dieting, preoccupation with weight, and entrenchment in an extreme pursuit of thinness), Bulimia (i.e., tendency toward episodes of binge eating that may be followed with the impulse to induce vomiting), and Body Dissatisfaction (i.e., belief that specific parts of the body are too large) subscales were used (Garner, Olmsted, & Polivy, 1983). Questions were phrased to indicate current disordered eating attitudes and behaviors. Only questions corresponding to these subscales were assessed in the TCHAD.
Although the EDI was created for use with female populations, it functions similarly in males. It can differentiate between males with EDs and controls (Olivardia et al., 1995) and produces the same factor structure and similar factor loadings and intercorrelations in college males and females (Spillane, Boerner, Anderson, & Smith, 2004). Reliability of the EDI in males is also acceptable (Eiben, 2007; Keel, Baxter, Heaterton, & Joiner, 2007; Keski-Rahkonen, Bulik, et al., 2005). Cronbach's alpha coefficients were previously reported for the identical sample used in the present study and were estimated at .88 and .81 for Drive for Thinness, .75 and .70 for Bulimia, and .91 and .88 for Body Dissatisfaction in females and males, respectively (Eiben, 2007).
Confirmatory factor analyses were conducted with Mplus 5.1 (Muthén & Muthén, 1998–2006) to examine the factor structure of the EDI items in our study's males and females. Initial confirmatory factor analyses indicated that the first question, “I eat candy and carbohydrates without worrying,” was a very poor indicator of its intended factor (Drive for Thinness) for both sexes and was excluded from further analysis. Model fits were assessed by examining the comparative fit index (CFI) and root mean square error of approximation (RMSEA). CFI values above .90 (Bentler, 1990) and RMSEA values from .05 to .08 indicate an acceptable fit (Browne & Cudeck, 1993). The original EDI factor solution reflected a reasonable fit for both females (CFI = .93, RMSEA = .07) and males (CFI = .95, RMSEA = .06).
Statistical Analyses
The EDI was scored as indicated by the EDI manual (Garner, 1991). Missing data were handled as follows: If subjects responded to more than 75% of items but less than 100% of items, missing values were imputed with the mean for that specific question. If fewer than 75% of the completed items were available, the score for the scale was considered missing. Thus, sample sizes vary across subscale analyses. Scores were normalized using SAS rank due to a positive skew, which assumes an underlying normal distribution of the observed scores. Items were created and normalized in this manner to remain consistent with previous research from TCHAD using the EDI (Eiben, 2007).
Univariate twin model-fitting analyses
The sources of variation in a trait that are decomposed in the classical twin design include additive genetic effects, shared environmental effects, and unique environmental effects. The unique environmental effects factor also includes measurement error. The basic principle of twin studies is that MZ twins are genetically identical whereas DZ twins are presumed to share, on average, 50% of their genes. However, recent research has shown that this assumption may be unreasonable, as several biological factors can result in functional genetic differences in MZ twins (Bruder et al., 2008; Gringras & Chen, 2001).
Our univariate twin model uses data from the five twin-zygosity groups: female–female MZ, female–female DZ, male–male MZ, male–male DZ, and male–female DZ. We fit the full sex-limitation twin model, allowing for qualitative and quantitative sex effects on normalized EDI scores. This model allows for the estimation of sex-dependent genetic and environmental parameters and also estimates the correlation between the genetic factors influencing disordered eating in males and females (rg).
Multivariate twin model-fitting analyses
All multivariate model-fitting analyses were conducted in males and females separately and then together, including in opposite-sex twins. First, an independent and common pathway model was fit to the normalized EDI data. Both models assume a common factor influences the observed variables but differ in the way the common factors influence variables. In the independent pathway model, the common genetic and environmental factors influence the observed variables directly with separate additive genetic, shared environmental, and unique environmental components estimated for each of the variable residuals. The common pathway model, however, asserts that a single latent factor underlies the observed variables. The variance in this factor is then partitioned into higher order additive genetic effects, shared environmental effects, and unique environmental effects. These components are also estimated for each observed variable's residual specifics. The common pathway model uses fewer parameters and is therefore more parsimonious than the independent pathway model. Therefore, if the common pathway model fits as well as the independent pathway model, it is preferred. Sex effects were examined as described above.
The Bayesian information criterion (BIC) was used to determine the best-fitting model. The BIC is a function of a model's degrees of freedom and chi-square goodness-of-fit (Raftery, 1995). Models that provide a better fit while retaining the fewest parameters yield lower BIC values. Twin modeling was done using a raw data approach in the statistical package Mx (M. C. Neale, 1997). This approach allows data from both incomplete and complete twin pairs to be used.
Although twin studies are a useful technique for exploring sex differences in the liability to a specific phenotype, their statistical power can be quite limited (M. C. Neale, Eaves, & Kendler, 1994). For phenotypes that are relatively rare in the population, exceptionally large samples are necessary to reliably detect sex differences in genetic risk factors (Prescott & Gottesman, 1993). Previous work by Sullivan and Eaves (2002) indicates that in studies with modest-sized samples with lower power, more valid parameter estimates are obtained using the full model than are obtained by the best-fitting model when constraining certain parameters to zero. Guided by this work, we only examine the full model here, which freely estimates both quantitative and qualitative sex effects.
Twin Model Assumptions
The twin method has three central assumptions that must be met. First, genetic effects are additive so that the genetic correlations for MZ and DZ twins are 1.0 and .50, respectively. Second, there is no assortative mating for the measure in question. Fortunately, there is little evidence for spousal similarity on most psychological characteristics (M. C. Neale et al., 1998). The third is referred to as the equal environments assumption. The equal environments assumption states that MZ and DZ twins are equally correlated for their exposure to environmental influences that are relevant to the trait under investigation. Empirical studies have found little evidence for violations of the equal environments assumption in EDs (Bulik et al., 2000; Klump, Holly, Iacono, McGue, & Willson, 2000).
Results
EDI
Sex differences in the EDI factor measurement structure were examined. First, using Mplus 5.1 (L. K. Muthén & Muthén, 2004), we conducted multiple group tests of measurement invariance between males and females for both factor loadings and thresholds for all indicators of the three EDI constructs. This was done by comparing models in which factor loadings and thresholds were constrained to be equal between groups with models allowing factor loadings and thresholds to vary by sex.
EDI data was recoded to binary for these measurement invariance tests. The invariance models took into account the nonindependence of twin structure. The Mplus robust weighted least squares mean and variance-adjusted estimator for categorical data was used to fit the measurement invariance models. Results produced a chi-square difference test of χ2(10, N = 2,368) = 34.5, p = .0002. However, the point estimates for the RMSEA and CFI for the unrestricted model (i.e., no invariance constraints) were .057 and .952, respectively, and these remained unchanged to the second decimal place under the restrictive factor loading and threshold measurement invariant model. Given the large sample size and associated power for testing this restrictive invariance hypothesis, these results collectively offer reasonable evidence that the three constructs are being measured in an equivalent manner across the sexes by the EDI items. Results are similar to a previous examination of the EDI using a smaller sample (Keel et al., 2007).
Finally, linear regression was used to assess the effects of sex on the subscales. Raw scores were converted to Z scores for ease of interpretability. Females had significantly higher scores on the Drive for Thinness subscale (β = 0.58, p < .001) and Body Dissatisfaction (β= 0.68, p < .001), with sex accounting for 29% and 33% of the variance, respectively. However, no difference was seen on Bulimia subscale scores, (β = .04, p = .35).
Descriptive Statistics
Table 1 presents the means, standard deviations, and intraclass correlations for the raw EDI scales. A significant mean difference was seen for female MZ and DZ twins for the Body Dissatisfaction subscale, with DZ twins scoring significantly higher ( p < .01). Overall, the pattern of correlations suggests genetic influences on all three subscales as all the observed correlations in MZ twins are higher than those observed in DZ twins. The correlations between opposite-sex twin pairs suggest that the familial factors influencing disordered eating are not entirely the same in the sexes because the observed correlations are lower than those observed in same-sex DZ pairs. However, these correlations are greater than zero, suggesting that some familial factors influence both male and female liability to disordered eating. Finally, with the exception of Bulimia in MZ pairs, the observed correlations were larger in female twin pairs. This would be expected if the specific traits were more heritable in females than in males.
Table 1. Means, Standard Deviations and Intraclass Correlations for Disordered Eating.
| Subscale | Females | Males | Opposite-sex twins | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||||||||
| All | MZ | DZ | All | MZ | DZ | Female | Male | ||||||||||||||
|
|
|
|
|
|
|
|
|
||||||||||||||
| M | SD | r | M | SD | r | M | SD | M | SD | r | M | SD | r | M | SD | r¥ | M | SD | M | SD | |
| Drive for Thinnessa | 2.13 | 3.90 | 0.61** | 2.00 | 4.00 | 0.32** | 2.36 | 4.01 | 0.36 | 1.40 | 0.30** | 0.31 | 1.43 | 0.23** | 2.24 | 4.00 | 0.12* | 5.90 | 6.5 | 0.34 | 1.34 |
| Bulimia | 0.54 | 1.51 | 0.30** | 0.50 | 1.40 | 0.23** | 0.51 | 1.12 | 0.49 | 1.27 | 0.33** | 0.43 | 1.2 | 0.15 | 0.44 | 1.15 | 0.14* | 0.62 | 2.00 | 0.55 | 1.34 |
| Body Dissatisfactiona | 5.74 | 6.20 | 0.64** | 5.04b | 5.90 | 0.35** | 6.24 | 6.21 | 2.20 | 3.60 | 0.45** | 1.93 | 3.30 | 0.28** | 2.24 | 4.00 | 0.10 | 5.90 | 6.50 | 2.44 | 3.67 |
Note. The means and standard deviations are for unstandardized scores. Drive for Thinness includes question dropped from twin analyses due to low loading on factor. MZ = monozygotic; DZ = dizygotic; r¥ = intraclass correlation between male and female of opposite-sex twin pair.
Significant mean difference between males and females on subscale.
Significant mean difference between MZ and DZ twins.
p < .05.
p < .01.
Univariate Twin Analyses
As seen in Table 2, model-fitting results indicate genetic factors influence disordered eating in both sexes. Heritabilities were estimated at 61% and 20% for Drive for Thinness, 16% and 33% for Bulimia, and 57% and 40% for Body Dissatisfaction for females and males, respectively. However, it is important to note the 95% confidence intervals for genetic effects in males for Drive for Thinness and Bulimia include zero. The full model provided genetic correlations between the sexes of .49, 1.00, and .66 for Drive for Thinness, Bulimia, and Body Dissatisfaction, respectively (see Table 3), suggesting that the genetic risk factors for Drive for Thinness and Body Dissatisfaction are not entirely the same in the sexes.
Table 2. Parameter Estimates for Univariate Models of Eating Disorder Inventory Subscales.
| Subscale | a2 | 95% CI | c2 | 95% CI | e2 | 95% CI |
|---|---|---|---|---|---|---|
| Drive for Thinness | ||||||
| Female | 0.61 | 0.33–0.68 | 0.01 | 0–0.25 | 0.38 | 0.31–0.46 |
| Male | 0.20 | 0–0.43 | 0.11 | 0–0.35 | 0.69 | 0.57–0.82 |
| Bulimia | ||||||
| Female | 0.16 | 0–0.42 | 0.16 | 0–0.36 | 0.69 | 0.57–0.81 |
| Male | 0.33 | 0–0.44 | 0 | 0–0.30 | 0.67 | 0.56–0.80 |
| Body Dissatisfaction | ||||||
| Female | 0.57 | 0.30–0.70 | 0.07 | 0–0.31 | 0.36 | 0.30–0.44 |
| Male | 0.40 | 0.06–0.57 | 0.07 | 0–0.35 | 0.53 | 0.44–0.64 |
Note. Total parameter estimates may be slightly above or below 1.00 due to rounding error. CI = confidence interval; a2 = additive genetic variance; c2 = shared environmental variance; e2 = unique environmental variance.
Table 3. Model-Fitting Results From Univariate and Multivariate Twin Models.
| Model | rg | df | −2LL | Δχ2 | p | BIC |
|---|---|---|---|---|---|---|
| Drive for Thinness | ||||||
| Saturated | 2171 | 4,600.20 | −5,358.77 | |||
| Sex effects | .49 | 2185 | 4,613.92 | 13.70 | .48 | −5,380.03 |
| Bulimia | ||||||
| Saturated | 2169 | 4,895.50 | −5,182.95 | |||
| Sex effects | 1.00 | 2177 | 4,910.54 | 15.04 | .06 | −5,203.50 |
| Body Dissatisfaction | ||||||
| Saturated | 2181 | 5,429.00 | −4,958.43 | |||
| Sex effects | .66 | 2189 | 5,435.00 | 6.00 | .65 | −4,983.58 |
| Multivariate common pathway | ||||||
| Sex effects | .71 | 6570 | 14,445.04 | −15,891.23 |
Note. For each subscale, the sex-effects model is compared with the saturated model. Saturated = in model fitting, the saturated model, which estimates all parameters, is used as a starting point for the comparison of different, nested models; Sex effects = model estimating sex-dependent parameters and genetic correlation; rg = genetic correlation; BIC = Bayesian information criterion.
Unique environmental influences are also important for all three subscales in both sexes. These estimates were fairly large for Drive for Thinness and Body Dissatisfaction in males as well as for Bulimia in both sexes, which could suggest a large amount of error in estimates. However, large estimates can also come from truly unique environmental effects, and we are unable to differentiate these two possibilities.
Multivariate Twin Analyses
In females, the common pathway model (BIC = −4,313.00) fit better than the independent pathway model (BIC = −4,304.74). Results were similar for males: The common pathway model (BIC = −4,267.33) fit better than the independent pathway model (BIC = −4,259.36).
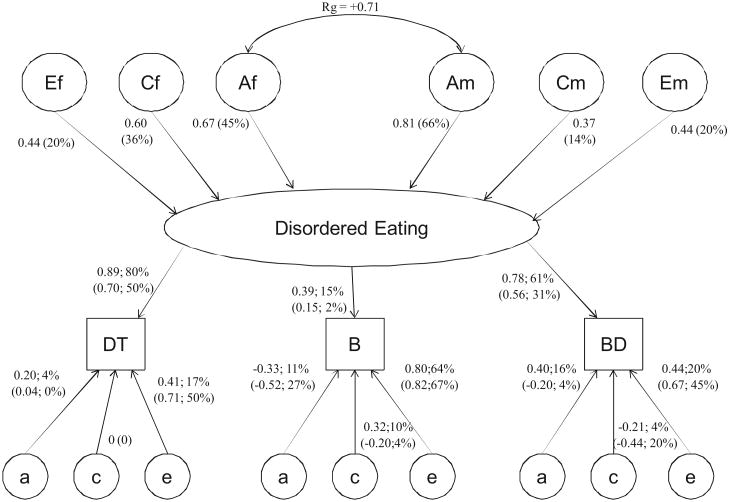
The full common pathway model estimated a genetic correlation of .71 between the latent liability for disordered eating in males and females (see Table 3 and Figure 1). By squaring the higher order path estimates from Figure 1 (represented by the abbreviations Af and Am), we estimated the heritability on this latent liability at 45% for females and 66% for males. However, when the proportion of genetic variance for each subscale is calculated, common genetic factors account for more of the variance in females for all subscales (see Table 4). Specifically, common genetic factors account for 36% (calculated by [0.67 × 0.89]2; see Figure 1) of the variance in Drive for Thinness scores for females and 32% in males, 7% of the variance in Bulimia scores for females and 1% in males, and 27% of the variance in Body Dissatisfaction scores in females and 21% in males.
Figure 1.
Genetic and environmental path estimates from multivariate common pathway model including all five zygosity groups. Rg = genetic correlation; Af = female common factor additive genetic path estimate; Cf = female common factor shared environmental path estimate; Ef = female common factor unique environment path estimate; Am = male common factor additive genetic path estimate; Cm = male common factor shared environmental path estimate; Em = male common factor unique environment path estimate; % = squared parameter estimates indicating percentage of variance accounted for by factor; DT = Drive for Thinness subscale; B = Bulimia subscale; BD = Body Dissatisfaction subscale; a = residual additive genetic path estimate; c = residual shared environmental path estimate; e = residual unique environment path estimate. Male path and parameter estimates for factor loadings and residual genetic and environmental factors are indicated in parentheses.
Table 4. Proportion of Variance Accounted for by Common and Specific Genetic and Environmental Factors in Females and Males From Multivariate Common Pathway Model Figure 1.
| Subscale | Genetic | Shared environment | Individual–specific (unique) environment | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| % common | % specific | Total | % common | % specific | Total | % common | % specific | Total | |
| Drive for Thinness | |||||||||
| Female | 36 | 4 | 39 | 29 | 0 | 29 | 15 | 17 | 32 |
| Male | 32 | 0 | 32 | 7 | 0 | 7 | 10 | 50 | 60 |
| Bulimia | |||||||||
| Female | 7 | 11 | 18 | 5 | 10 | 15 | 3 | 64 | 67 |
| Male | 1 | 27 | 28 | 0 | 4 | 4 | 0 | 67 | 67 |
| Body Dissatisfaction | |||||||||
| Female | 27 | 16 | 43 | 22 | 4 | 26 | 12 | 20 | 32 |
| Male | 21 | 4 | 25 | 4 | 20 | 24 | 6 | 45 | 51 |
Note. Total common and specific genetic and environmental proportions may be slightly above or below 100% due to rounding error.
Although the higher heritability for the latent factor in males compared with females may seem contradictory, the total heritability for each of the subscales (including the proportion of genetic variance from the common factor, as well as the proportion specific to the subscale) is higher in females for all three subscales. The fact that the heritability for the latent factor is higher in males versus females reflects the fact that the genetic factors that the three subscales have in common explain more of the variance of the latent factor in males but are partly different from those in females (rg = .71). However, a smaller proportion of the variance of each of the subscales is accounted for by the common factor in males than in females (lower factor loadings).
As can be seen in Table 4, common shared and unique environmental factors also account for more of the variance in females than in males for all subscales. Common shared and unique environmental variance was greatest for Drive for Thinness at 29% ([0.60 × 0.89]2; see Figure 1) and 15% ([0.44 × 0.89]2; see Figure 1), respectively. Males have substantially more variable specific unique environmental influences for all subscales. The greatest amount of specific unique environmental influence was estimated at 67% for the Bulimia subscale. This statistic can be calculated by squaring the subscale, sex-specific residual path estimate (see Figure 1). For Bulimia, this is calculated by squaring 0.82.
Several results from these analyses are noteworthy. First, the genetic risk factors for the disordered eating common factor are not entirely the same in both sexes. Second, common genetic factors account for more of the variance in this factor for females than for males. Third, the loadings for all three subscales on the common factor were larger for females than for males. Fourth, Bulimia is the least discriminating subscale and Drive for Thinness the most salient indicator of the factor in both sexes.
Discussion
We had three aims for this investigation: (a) to examine the structure of disordered eating (i.e., whether a single common factor accounts for the association among the three disordered eating facets), (b) to examine quantitative and qualitative differences on this possible common factor; and (c) to examine quantitative and qualitative differences in three specific facets of disordered eating. Findings associated with each of these aims are discussed.
Structure of Disordered Eating
To our knowledge, only two previous studies have examined the factor structure of the genetic and environmental influences on disordered eating, and both indicate a single underlying factor is responsible (B. M. Neale et al., 2003; Wade et al., 1999). Results of the current study corroborate and extend these findings by indicating this structure is quite similar in males and females. However, our three subscales tend to discriminate individual differences more sharply on the factor for females than they do in males. This is not surprising given that the EDI was developed for use with a female population (Garner, 1991).
Of note, the Bulimia subscale was a poor indicator of this latent liability. The common factor only accounted for 15% (0.392) and 2% (0.152) of the variance in Bulimia scores for females and males, respectively (see Figure 1). Similarly, however, the two previously discussed studies also had a variable—specifically, a measure of restraint (B. M. Neale et al., 2003) and a semi-structured ED interview (Wade et al., 1999)—for which the latent factor did not account for a large proportion of variance.
Sex Differences in Disordered Eating
Quantitative sex effects
Our modeling produced evidence for quantitative sex effects. Genetic factors showed a stronger contribution in males, whereas shared environment contributed more in females when accounting for variability in latent liability to disordered eating. Unique environmental factors appear to be contributing at an equal magnitude in the sexes. Quantitative effects were also revealed at the univariate level for Drive for Thinness and Body Dissatisfaction. Heritability estimates were greater for females than for males, and 95% confidence intervals also indicate lower genetic variability. These results are similar to those from previous research by Slane et al. (2007), whose population age and sample size were similar to those in the present study.
Qualitative sex effects
Only two previous reports, both examining binge eating, have examined qualitative sex differences in disordered eating. Our results suggest that the genetic risk factors for disordered eating in general, and particularly for Drive for Thinness and Body Dissatisfaction, are not entirely the same in males and females. Only approximately 50% of the genetic risk factors are shared between the sexes.
In contrast, our univariate results indicate all of the genetic risk factors for bulimia are shared. This is discrepant with previous reports examining binge eating (Reichborn-Kjennerud et al., 2003; Reichborn-Kjennerud, Bulik, Tambs, & Harris, 2004). Although binge eating alone was not assessed in the current study, the Bulimia subscale would be the most comparable. Reichborn-Kjennerud et al. (2003) showed a very slight improvement in model fit when the genetic correlation was allowed to be estimated, revealing a genetic correlation of .57. However, the results of our multivariate model do provide evidence of qualitative differences on Bulimia, as the genetic risk for the disordered eating factor does not completely overlap between the sexes.
This discrepant result might arise from several methodological differences between the studies. First, our study had a considerably smaller sample size, so with more power, we might have detected these qualitative sex effects at the univariate level. Second, the age range for participants in the current study (15–17 years) is younger than the ages of the participants in the study of Reichborn-Kjennerud et al. (19 –31 years; Reichborn-Kjennerud, Bulik, Kendler, et al., 2004; Reichborn-Kjennerud, Bulik, Tambs, & Harris, 2004). Differences in assessment could also have impacted results. For example, our measures examine aspects of disordered eating that relate to EDs, whereas the previous study used questions designed to capture DSM–IV criteria for binge eating. We also used a Swedish population, whereas the former was a Norwegian population. However, these two populations are culturally similar.
Candidates for Sex Differences in Disordered Eating
Our study provided evidence for qualitative sex differences in disordered eating but no direct information about the nature of these differences. Several plausible hypotheses are worth considering. First, results are consistent with the hypothesis that gonadal hormone exposure creates a differential ED risk in males and females (Klump et al., 2005; Reichborn-Kjennerud et al., 2003). For example, research indicates estrogen may play an important role in disordered eating (e.g., Klump, Burt, Sisk, & Keel, 2007; Klump et al., 2005). Disordered eating in females is also related to effects of cyclic hormonal changes (Edler et al., 2006; Lester, Keel, & Lipson, 2003), which would not be expressed in males.
Similarly, testosterone levels could impact disordered eating. Decreased levels of testosterone have previously been linked to AN in males (APA, 2000). Testosterone has also been shown to be a protective factor against disordered eating in females (Culbert, Breedlove, Burt, & Klump, 2008). Because testosterone levels decline in males as they age, especially after the age of 40 years (e.g., Feldman et al., 2002), one can hypothesize that as testosterone levels decrease, males become more susceptible to disordered eating. However, testosterone levels in males with AN improve with weight gain (Scott, 1986), and studies have been unable to replicate results suggesting that testosterone impacts risk for disordered eating (Baker, Lichtenstein, & Kendler, 2009; Raevuori et al., 2008).
Finally, several social and psychological factors relevant to EDs could be the basis for this sex difference in genetic risk. For example, cultural pressure to be thin is much greater for females than for males. Additional psychological factors such as childhood sexual abuse, personality characteristics, and symptoms of depression or anxiety may also play a role. These differing social and psychological factors could produce separate pathways to ED development in males and females, thereby creating a distinct genetic variation in risk. For example, given the different cultural influences on body shape in males and females, the kinds of temperamental effects that impact on body shape could differ across sexes. This, in turn, would create different genetic effects, driving males to be more muscular and females to be thin and have small waists.
Study Limitations and Future Directions
Several limitations warrant discussion. One possible limitation is our sample size and the associated modest statistical power, especially for the detection of sex effects. Our sample also comes from a single birth cohort in Sweden. Although studies suggest the prevalence of EDs in Sweden and other Scandinavian countries is similar to slightly less prevalent compared with the prevalence of EDs the United States, we still cannot be certain that our findings would extrapolate to other populations (Ghaderi & Scott, 2001; Rastam, Gillberg, & Garton, 1989).
There are also limitations to our use of the EDI. First, because of our low mean scores, especially within the Bulimia subscale, there may not have been adequate variability to detect sex differences at the univariate level. Second, males and females likely have differential thresholds for expressing disordered eating, which would impact results. For example, the Bulimia subscale may represent more normative aspects of behaviors in males (Eiben, 2007). Many of the subscale items deal with binge eating behaviors, and 15- to 17-year-old boys commonly consume large amounts of food (Eiben, 2007; Katzman, Wolchik, & Braver, 1984). Males are also less likely to label the consumption of large quantities of food a binge or to report feeling out of control during the food consumption (Carlat & Camargo, 1991; Franco, Tamburino, Carroll, & Bernal, 1988; Lewinsohn et al., 2002). However, results from our measurement invariance analyses provide some limited evidence that this is not the case, as we constrained item-level thresholds to be equal; we did the same for factor loadings. Finally, as previously noted, males typically desire to be more muscular whereas females desire to be smaller, and the Body Dissatisfaction subscale of the EDI focuses on the latter. The Body Dissatisfaction subscale also focuses on core areas of the body with which females are more likely to express dissatisfaction (i.e., stomach and thighs).
We found several differences in the genetic risk factors for disordered eating in males and females. However, replication of this study with a larger sample size and at different ages is needed to make definitive conclusions about etiologic differences in disordered eating, as our results are limited to adolescence. Future research should also examine genetic and shared environmental similarities and differences in AN and BN diagnoses, and it should focus on examining developmental trajectories of disordered eating in males as it is possible genetic and environmental influences change across development.
Acknowledgments
This research and a portion of the manuscript preparation were supported by Grant T32-DA-007027 from the National Institute on Drug Abuse to Jessica H. Baker. This research was supported by funds from the Swedish Council for Working Life and Social Research to Paul Lichtenstein and Lauren Lissner and the Swedish Research Council to Paul Lichtenstein. A portion of manuscript preparation was also supported by Grant T32 MH020030 from the National Institutes of Health to Jessica H. Baker. Additional funding came from Grant R01 DA018673, the Virginia Tobacco Settlement Foundation, Grant R01 DA022989, and Grant U01 DA024413 to Hermine H. Maes.
Footnotes
Portions of this article were presented at the International Conference on Eating Disorders, May 2008, Seattle, Washington.
Contributor Information
Jessica H. Baker, Department of Psychology and Virginia Institute for Psychiatric and Behavioral Genetics, Department of Psychiatry, Medical College of Virginia, Virginia Commonwealth University
Hermine H. Maes, Department of Human Genetics, Massey Cancer Center, and Virginia Institute for Psychiatric and Behavioral Genetics, Department of Psychiatry, Medical College of Virginia, Virginia Commonwealth University
Lauren Lissner, Department of Public Health and Community Medicine, Primary Health Care, Sahlgrenska Academy, Göteborg University, Göteborg, Sweden.
Steven H. Aggen, Virginia Institute for Psychiatric and Behavioral Genetics, Department of Psychiatry, Medical College of Virginia, Virginia Commonwealth University
Paul Lichtenstein, Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Solna, Sweden.
Kenneth S. Kendler, Virginia Institute for Psychiatric and Behavioral Genetics, Department of Psychiatry, Medical College of Virginia, Virginia Commonwealth University
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th, text rev. Washington DC: Author; 2000. [Google Scholar]
- Andersen AE. Eating disorders: A guide to medical care and complications. In: Mehler MS, Andersen AE, editors. Males with eating disorders: Medical considerations. Baltimore: Johns Hopkins University Press; 1999. pp. 214–225. [Google Scholar]
- Andersen AE, Holman JE. Males with eating disorders: Challenges for treatment and research. Psychopharmacology Bulletin. 1997;33:391–397. [PubMed] [Google Scholar]
- Anderson CB, Bulik CM. Sex differences in compensatory behaviors, weight and shape salience, and drive for thinness. Eating Behaviors. 2004;5:1–11. doi: 10.1016/j.eatbeh.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Baker JH, Lichtenstein P, Kendler KS. Intrauterine testosterone exposure and risk for disordered eating. British Journal of Psychiatry. 2009;194:375–376. doi: 10.1192/bjp.bp.108.054692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bosch-Bramon E, Troop NA, Treasure JL. Eating disorders in males: A comparison with female patients. European Eating Disorders Review. 2000;8:321–328. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Thousand Oaks, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Bruder CEG, Piotrowski A, Gijsbers AACJ, Andersson R, Erickson S, Diaz de Ståhl T, et al. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. The American Journal of Human Genetics. 2008;82:763–771. doi: 10.1016/j.ajhg.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Society of Biological Psychiatry. 1998;44:1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Archives of General Psychiatry. 2006;63:305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Wade T, Kendler KS. Twin studies of eating disorders: A review. International Journal of Eating Disorders. 2000;27:1–20. doi: 10.1002/(sici)1098-108x(200001)27:1<1::aid-eat1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Carlat DJ, Camargo CA., Jr Review of bulimia nervosa in males. American Journal of Psychiatry. 1991;148:831–843. doi: 10.1176/ajp.148.7.831. [DOI] [PubMed] [Google Scholar]
- Carlat DJ, Camargo CA, Jr, Herzog DB. Eating disorders in males: A report on 135 patients. American Journal of Psychiatry. 1997;154:1127–1132. doi: 10.1176/ajp.154.8.1127. [DOI] [PubMed] [Google Scholar]
- Cohane GH, Pope HG., Jr Body image in boys: A review of the literature. International Journal of Eating Disorders. 2001;29:373–379. doi: 10.1002/eat.1033. [DOI] [PubMed] [Google Scholar]
- Crisp AH, Burns T, Bhat AV. Primary anorexia nervosa in the male and female: A comparison of clinical features and prognosis. British Journal of Medical Psychology. 1986;59:123–132. doi: 10.1111/j.2044-8341.1986.tb02676.x. [DOI] [PubMed] [Google Scholar]
- Croll J, Neumark-Sztainer D, Story M, Ireland M. Prevalence and risk and protective factors related to disordered eating behaviors among adolescents: Relationship to gender and ethnicity. Journal of Adolescent Health. 2002;31:166–175. doi: 10.1016/s1054-139x(02)00368-3. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: A comparison of opposite-sex and same-sex twins. Archives of General Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine. 2006;37:131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Eiben G. Unpublished doctoral thesis. Göteborg University; Göteborg, Sweden: 2007. Overweight and obesity in the young and old: Prevalence, prevention, and eating behavior. [Google Scholar]
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araugo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts Male Aging Study. Journal of Clinical Endocrinology and Metabolism. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- Franco KSN, Tamburino MB, Carroll BT, Bernal GAA. Eating attitudes in college males. International Journal of Eating Disorders. 1988;7:285–288. [Google Scholar]
- Garner DM. Eating Disorder Inventory–2: Professional manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Garner DM, Olmsted MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. International Journal of Eating Disorders. 1983;2:15–34. [Google Scholar]
- Geist R, Heinmaa M, Katzman D, Stephens D. A comparison of male and female adolescents referred to an eating disorder program. Canadian Journal of Psychiatry. 1999;44:374–378. doi: 10.1177/070674379904400408. [DOI] [PubMed] [Google Scholar]
- Ghaderi A, Scott B. Prevalence, incidence and prospective risk factors for eating disorders. Acta Psychiatrica Scandinavica. 2001;104:122–130. doi: 10.1034/j.1600-0447.2001.00298.x. [DOI] [PubMed] [Google Scholar]
- Gringras P, Chen W. Mechanisms for differences in monozygous twins. Early Human Development. 2001;64:105–117. doi: 10.1016/s0378-3782(01)00171-2. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope H, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: Application of risk terminology and suggestions for a general taxonomy. Psychological Bulletin. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- Katzman MA, Wolchik SA, Braver SL. The prevalence of frequent binge eating and bulimia in a nonclinical college sample. International Journal of Eating Disorders. 1984;3:53–62. [Google Scholar]
- Keel PK, Baxter MG, Heaterton TF, Joiner TE. A 20-year longitudinal study of body weight, dieting, and eating disorder symptoms. Journal of Abnormal Psychology. 2007;116:422–432. doi: 10.1037/0021-843X.116.2.422. [DOI] [PubMed] [Google Scholar]
- Kendler KS, MacLean C, Neale MC, Kessler RC, Heath AC, Eaves LJ. The genetic epidemiology of bulimia nervosa. American Journal of Psychiatry. 1991;148:1627–1637. doi: 10.1176/ajp.148.12.1627. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, environment, and psychopathology: Understanding the causes of psychiatric and substance use disorders. New York: Guilford Press; 2006. [Google Scholar]
- Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Archives of General Psychiatry. 1995;52:374–383. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- Keski-Rahkonen A, Bulik CM, Neale BM, Rose RJ, Rissanen A, Kaprio J. Body dissatisfaction and drive for thinness in young adult twins. International Journal of Eating Disorders. 2005;37:188–199. doi: 10.1002/eat.20138. [DOI] [PubMed] [Google Scholar]
- Keski-Rahkonen A, Neale BM, Bulik CM, Pietiläinen KH, Rose RJ, Kaprio J, Rissanen A. Intentional weight loss in young adults: Sex-specific genetic and environmental effects. Obesity Research. 2005;13:745–753. doi: 10.1038/oby.2005.84. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, Sisk C, Keel PK. Estrogen moderates genetic effects on disordered eating during puberty; Paper presented at the 13th Annual Meeting of the Eating Disorders Research Society; Pittsburgh, PA. 2007. Oct, [Google Scholar]
- Klump KL, Gobrogge KL, Perkins PS, Thorne D, Sisk CL, Breedlove M. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychological Medicine. 2005;36:539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- Klump KL, Holly A, Iacono WG, McGue M, Willson LE. Physical similarity and twin resemblance for eating attitudes and behaviors: A test of the equal environments assumption. Behavior Genetics. 2000;24:215–225. doi: 10.1023/a:1002038610763. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: Exploring associations in community samples. Psychological Medicine. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Miller KB, Keel PK, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychological Medicine. 2001;31:737–740. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]
- Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: Relation to menstrual-cycle phase and cortisol levels. Psychological Medicine. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Moerk KC, Striegel-Moore RH. Gender differences in eating disorder symptoms in young adults. International Journal of Eating Disorders. 2002;32:426–440. doi: 10.1002/eat.10103. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Svartengren M. Genes, environments, and sex: Factors of importance in atopic diseases in 7–9-year-old Swedish twins. Allergy. 1997;52:1079–1086. doi: 10.1111/j.1398-9995.1997.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Tuvblad C, Larsson H, Carlström E. The Swedish Twin Study of CHild and Adolescent Development: The TCHAD-Study. Twin Research and Human Genetics. 2007;10:67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- Mazzeo SE, Mitchell KS, Bulik CM, Reichborin-Kjennerud T, Kendler KS, Neale MC. Assessing the heritability of anorexia nervosa symptoms using a marginal maximal likelihood approach. Psychological Medicine. 2008;19:1–11. doi: 10.1017/S0033291708003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Blissett J, Oldfield C. Sexual orientation and eating psychopathology: The role of masculinity and femininity. International Journal of Eating Disorders. 2001;29:314–318. doi: 10.1002/eat.1024. [DOI] [PubMed] [Google Scholar]
- Muise AM, Stein DG, Arbess G. Eating disorder in adolescent boys: A review of the adolescent and young adult literature. Journal of Adolescent Health. 2003;33:427–525. doi: 10.1016/s1054-139x(03)00060-0. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén B. Mplus user's guide. Vol. 4. Los Angeles: Muthén & Muthén; 1998–2006. [Google Scholar]
- Muthén LK, Muthén BO. Mplus: The comprehensive modeling program for applied researchers: User's guide. 3rd. Los Angeles: Muthén & Muthén; 2004. [Google Scholar]
- Neale BM, Mazzeo SE, Bulik CM. A twin study of dietary restraint, disinhibition and hunger: An examination of the Eating Inventory (Three Factor Eating Questionnaire) Twin Research and Human Genetics. 2003;6:471–478. doi: 10.1375/136905203322686455. [DOI] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical modeling. 2nd. Richmond, VA: Medical College of Virginia, Department of Psychiatry; 1997. [Google Scholar]
- Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behavior Genetics. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- Neale MC, Kendler KS, Hewitt JK, Silberg JL, Foley DL, Meyer JM, et al. Assortative mating for major psychiatric diagnoses in two population-based samples. Psychological Medicine. 1998;28:1389–1401. doi: 10.1017/s0033291798007326. [DOI] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Hannan PJ. Weight-related behaviors among adolescent girls and boys: Results from a national survey. Archives of Pediatric and Adolescent Medicine. 2000;154:569–577. doi: 10.1001/archpedi.154.6.569. [DOI] [PubMed] [Google Scholar]
- Nevonen L, Clinton D, Norring C. Validating the EDI-2 in three Swedish female samples: Eating disorder patients, psychiatric outpatients and normal controls. Nordic Journal of Psychiatry. 2006;60:44–50. doi: 10.1080/08039480500504537. [DOI] [PubMed] [Google Scholar]
- Norring C, Sohlberg S. Eating Disorder Inventory in Sweden: Description, cross-cultural comparison, and clinical utility. Acta Psychiatrica Scandinavica. 1988;78:567–575. doi: 10.1111/j.1600-0447.1988.tb06386.x. [DOI] [PubMed] [Google Scholar]
- Olivardia R, Pope H, Jr, Mangweth B, Hudson J. Eating disorders in college men. American Journal of Psychiatry. 1995;152:1279–1285. doi: 10.1176/ajp.152.9.1279. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Gottesman II. Power limitations in detecting heterogeneity of genetic effects: The case of sex differences in alcoholism; Paper presented at the annual meeting of the Society for Research on Psychopathology; Chicago. 1993. Oct, [Google Scholar]
- Presnell K, Bearman SK, Stice E. Risk factors for body dissatisfaction in adolescent boys and girls: A prospective study. International Journal of Eating Disorders. 2004;36:389–401. doi: 10.1002/eat.20045. [DOI] [PubMed] [Google Scholar]
- Raevuori A, Kaprio J, Hoek HW, Sihvola E, Rissanen A, Keski-Rahkonen A. Anorexia and bulimia nervosa in same-sex and opposite-sex twins: Lack of association with twin type in a nationwide study of Finnish twins. American Journal of Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.08030362. Advance online publication. Retrieved December 17, 2008. [DOI] [PubMed] [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociological Methodology. 1995;25:111–163. [Google Scholar]
- Rastam M, Gillberg C, Garton M. Anorexia nervosa in a Swedish urban region. British Journal of Psychiatry. 1989;155:642–646. doi: 10.1192/s0007125000018134. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Kendler KS, Roysamb E, Maes H, Tambs K, et al. Sex differences in binge eating: A population-based twin study. Acta Psychiatrica Scandinavica. 2003;108:196–202. doi: 10.1034/j.1600-0447.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Kendler KS, Roysamb E, Tambs K, Torgersen S, et al. Undue influence of weight on self-evaluation: A population-based twin study of gender differences. International Journal of Eating Disorders. 2004;35:123–132. doi: 10.1002/eat.10252. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Tambs K, Harris JR. Genetic and environmental influences on binge eating in the absence of compensatory behaviors: A population-based twin study. International Journal of Eating Disorders. 2004;36:307–314. doi: 10.1002/eat.20047. [DOI] [PubMed] [Google Scholar]
- Ricciardelli LA, McCabe MP. Dietary restraint and negative affect as mediations of body dissatisfaction and bulimic behaviors in adolescent girls and boys. Behaviour Research and Therapy. 2001;39:1317–1328. doi: 10.1016/s0005-7967(00)00097-8. [DOI] [PubMed] [Google Scholar]
- Ricciardelli LA, McCabe MP. A biopsychosocial model of disordered eating and the pursuit of muscularity in adolescent boys. Psychological Bulletin. 2004;130:179–205. doi: 10.1037/0033-2909.130.2.179. [DOI] [PubMed] [Google Scholar]
- Russell CJ, Keel PK. Homosexuality as a specific risk factor for eating disorders in men. International Journal of Eating Disorders. 2002;31:300–306. doi: 10.1002/eat.10036. [DOI] [PubMed] [Google Scholar]
- Scott DW. Anorexia nervosa in the male: A review of clinical epidemiological and biological findings. International Journal of Eating Disorders. 1986;5:799–819. [Google Scholar]
- Slane J, Burt A, Klump KL. Sex similarities in genetic and environmental influences on disordered eating; Paper presented at the 13th Annual Meeting of the Eating Disorders Research Society; Pittsburgh, PA. 2007. Oct, [Google Scholar]
- Spillane NS, Boerner LM, Anderson KG, Smith GT. Comparability of the Eating Disorder Inventory–2 between women and men. Assessment. 2004;11:85–93. doi: 10.1177/1073191103260623. [DOI] [PubMed] [Google Scholar]
- Stice E. Review of the evidence for a sociocultural model of bulimia nervosa and exploration of mechanisms of action. Clinical Psychology Review. 1994;14:633–661. [Google Scholar]
- Sullivan PF, Eaves LJ. Evaluation of analyses of univariate discrete twin data. Behavior Genetics. 2002;32:221–227. doi: 10.1023/a:1016025229858. [DOI] [PubMed] [Google Scholar]
- Tholin S, Rasmussen F, Tynelius P, Karlsson J. Genetic and environmental influences on eating behaviors: The Swedish Young Male Twins Study. American Journal of Clinical Nutrition. 2005;81:564–569. doi: 10.1093/ajcn/81.3.564. [DOI] [PubMed] [Google Scholar]
- Wade TD, Bulik CM, Kendler KS. Anorexia nervosa and major depression: An examination of shared genetic and environmental risk factors. American Journal of Psychiatry. 2000;157:469–471. doi: 10.1176/appi.ajp.157.3.469. [DOI] [PubMed] [Google Scholar]
- Wade TD, Martin NG, Neale MC, Tiggemann M, Treloar SA, Bucholz KK, et al. The structure of genetic and environmental risk factors for three measures of disordered eating. Psychological Medicine. 1999;29:925–934. doi: 10.1017/s0033291799008740. [DOI] [PubMed] [Google Scholar]
- Woodside DB, Garfinkel PE, Lin E, Goering P, Kaplan AS, Goldbloom DS, et al. Comparisons of men with full or partial eating disorders, men without eating disorders, and women with eating disorders in the community. American Journal of Psychiatry. 2001;158:570–574. doi: 10.1176/appi.ajp.158.4.570. [DOI] [PubMed] [Google Scholar]