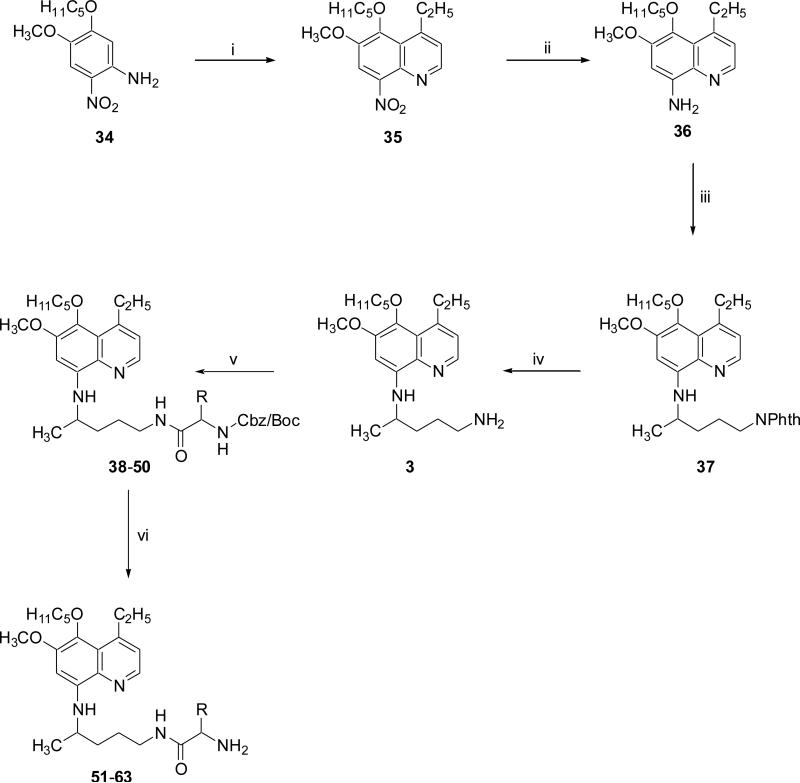
Scheme 2. Reagents and conditions.
(i) 1-chloro-3-pentanone, 85% o-H3PO4, As2O5, 80 °C, 3 h; (ii) raney nickel, EtOH, H2, 45 psi, 45 min; (iii) 2-(4-bromopentyl)-1,3-isoindolinedione, Et3N, 120 °C, 24 h; (iv) NH2NH2.H2O, EtOH, reflux, 8 h; (v) suitably N- and side-chain protected L/D-amino acid, DCC, DCM, rt, 6 h; (vi) H2/Pd-C, MeOH, 1h, rt or 4N HCl-MeOH, 1h, rt or 30% HBr-AcOH, 45 min, rt.