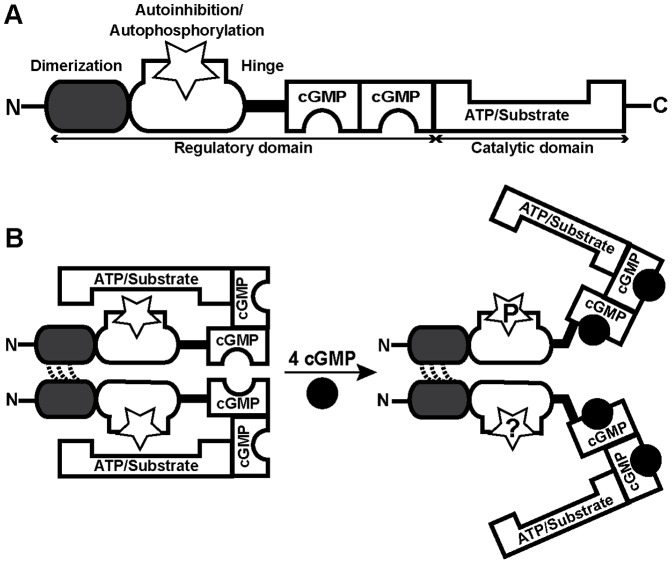
Figure 1. General structure and current working model of cGKI.
(A) cGKI consists of a C-terminal catalytic domain and an N-terminal regulatory domain. The catalytic domain contains binding sites for ATP and protein substrates with Ser/Thr residues. The regulatory domain comprises two non-identical cGMP-binding pockets and additional regions with multiple functions: a leucine zipper for dimerization of two identical subunits, an overlapping autoinhibitory/autophosphorylation region (open star), and a flexible hinge region connecting the N-terminal region to the rest of the protein. (B) According to the current model, the homodimeric enzyme cannot heterophosphorylate substrates in the absence of cGMP (left). Binding of cGMP (black circles) results in a conformational change that allows heterophosphorylation of substrates (right). According to in vitro studies with purified cGKI, the N-terminal region of the inactive kinase is not phosphorylated (left, stars), and activation is associated with autophosphorylation of distinct sites in this region (right, star with a “P”). However, it is not clear whether or not N-terminal phosphorylation of cGKI does also occur in intact cells (right, star with a “?”).