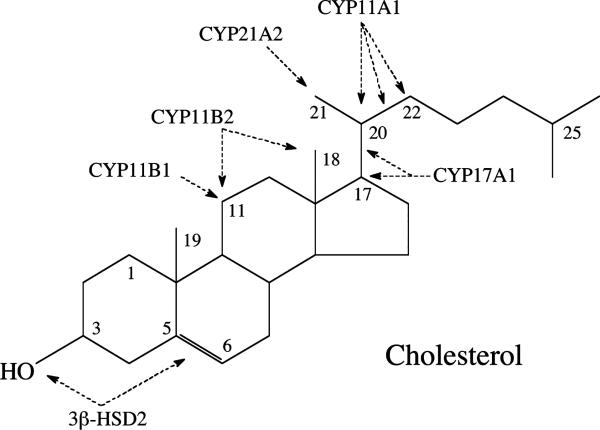
Figure 1.
The sites of modification of the steroid molecule by the steroidogenic enzymes present in the adrenal cortex. The sites of metabolism, using the cholesterol molecule as a template, are shown by arrows (for further details see Fig. 2). Note that the enzymes act in a specific order, dictated by the structure of their immediate substrate. Only CYP11A1 can act on cholesterol, cleaving six carbons from the side chain to produce pregnenolone (23,25). This reaction involves initial hydroxylation of the side chain at C22 to produce 22R-hydroxycholesterol, hydroxylation at C20 to produce 20R,22R-dihydroxycholesterol then oxidative cleavage of the C20-C22 bond producing pregnenolone and isocaproic aldehyde (25,27).