Abstract
The priming effect refers to quantitative changes in microbial decomposition of recalcitrant organic matter upon addition of labile organic matter and is a phenomenon that mainly has been reported and debated in soil science. Recently, priming effects have been indicated in aquatic ecosystems and have received attention due to the potential significance for ecosystem carbon budgets. Headwater stream biofilms, which are important degraders of both allochthonous, presumably recalcitrant, organic matter and labile autochthonous organic matter, may be sites where priming effects are important in aquatic environments. We have experimentally tested for priming effects in stream biofilms within microcosms mimicking the stream hyporheic zone. A 13C labeled model allochthonous carbon source was used in combination with different carbon sources simulating autochthonous inputs. We did not detect changes in respiration, removal or incorporation of allochthonous organic matter in response to autochthonous treatments, thus not supporting the occurrence of priming effects under the experimental conditions. This study is the first to address priming effects in the hyporheic zone, and one of very few studies quantitatively assessing aquatic priming effects. The results contrast with existing studies, which highlights the need for quantitative approaches to determine the importance of priming effects in aquatic environments.
The hyporheic zone of streams is a site of intense organic matter processing carried out by microorganisms colonizing sediment particles1,2. A defining feature of the hyporheic zone is the mixing of waters containing organic matter (OM) of diverse origin and bioavailability. Allochthonous OM, originating from terrestrial primary production can enter the streams for example via leaf litter fall and surface water runoff that carries plant material and allochthonous dissolved OM (DOM) into streams. These allochthonous inputs may reach the hyporheic zone via downwelling flow or physical disturbance of the sediments. In addition, groundwater welling up through the hyporheic zone supplies allochthonous DOM originating from soils and aquifers to streams. Alongside allochthonous OM, autochthonous OM can also be transported to the hyporheic zone from the adjacent benthic zone3. A large portion of autochthonous primary production by benthic algae is eventually converted to DOM through exudation, lysis and decomposition of algal cells. Autochthonous DOM contains carbohydrates, amino acids and proteins, making it easily available to microbial heterotrophs. On the other hand, allochthonous DOM is typically processed by microorganisms before it enters streams, resulting in a highly complex mix of molecules that differ in size, age and bioavailability4,5,6.
In soils, it has been repeatedly demonstrated that supply of bioavailable OM can stimulate the microbial degradation of preexisting soil OM7. This is referred to as the priming effect8 (PE), which can be defined as quantitative changes in the degradation of so-called recalcitrant OM by microbial communities in the presence of labile OM. PE can be either positive or negative, though a surplus degradation of recalcitrant OM upon the addition of labile OM (i.e. positive PE) is most often discussed. After decades of PE research in soils, it has been concluded that quantifying PE and distinguishing between real and apparent PE requires separating the contribution of different carbon pools to the total metabolism using for example stable isotope labeling approaches9.
In recent years PE has been highlighted as potentially important not only in soils, but also in aquatic ecosystems10,11. Guenet and colleagues10 suggested that there may be “hotspots” and “hot moments” in aquatic ecosystems where PE could be especially significant, because of co-occurrence of labile- and recalcitrant OM sources. In streams, hotspots of PE may be found in sites where allochthonous material that has been depleted in bioavailable OM meets autochthonous inputs from benthic primary producers, such as in the hyporheic zone.
The fate of allochthonous OM as it enters the aquatic realm has profound implications for carbon cycling on landscape and global scales. Whether it is mineralized, buried through sedimentation or transported downstream determines if aquatic ecosystems are net sources or sinks of CO212. Currently, it is recognized that headwater streams are generally net sources of CO2 due to intensive mineralization of allochthonous OM13, yet the mechanisms that enable this rapid turnover remain elusive14. PE may indeed be one such mechanism, and knowledge of the occurrence and magnitude of PE is therefore critically important in order to understand the roles of streams and other aquatic ecosystems in global carbon budgets. Still, there are few studies addressing PE in aquatic ecosystems and even fewer have used a quantitative stable isotope approach.
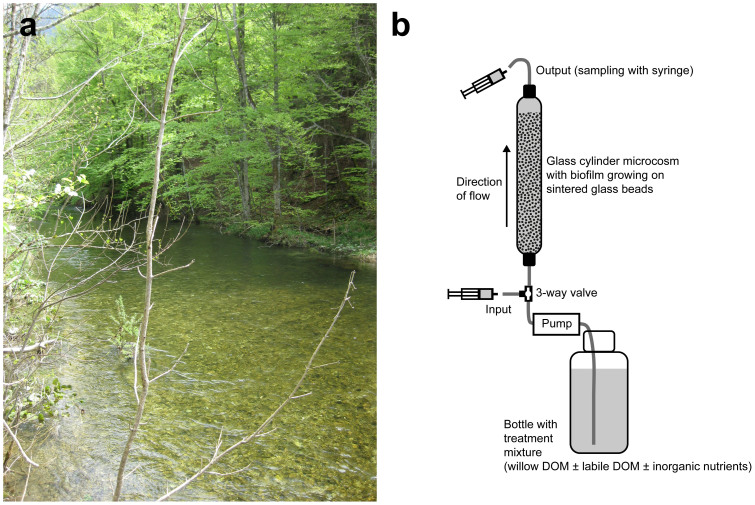
In this study, we investigated the occurrence and magnitude of PE in the hyporheic zone by testing the effect of labile carbon additions simulating autochthonous DOM on the microbial degradation of allochthonous DOM. We used a microcosm approach, featuring plug-flow bioreactors colonized by hyporheic microbial communities from an oligotrophic pre-alpine stream (Fig. 1). The microcosms were amended with a pre-degraded, 13C-labeled plant extract, made from willow (Salix fragilis). It was designed to be similar to microbially processed leaf litter DOM, an important type of allochthonous DOM in headwater streams. The 13C label allowed accurate quantification of respiration, removal and biomass incorporation of this model allochthonous DOM source. Pulsed additions of different combinations of glucose, inorganic nutrients and an algal extract simulated inputs of labile autochthonous DOM, as for example during an algal bloom.
Figure 1.
The Oberer Seebach, from where the hyporheic microbial communities and stream water were sampled (a). The setup of the microcosms used in the study (b).
Results
Allochthonous DOM metabolism and priming effect (PE)
There were no significant differences in respiration and removal of the 13C-labeled model allochthonous DOM (willow DOM) during additions of labile DOM (pulse phase, Fig. 2a–3a), or after the labile DOM additions were stopped (post-pulse phase, Fig. 2b–3b). The only exception is a slightly, yet significantly, lower portion of willow DOM removed when glucose in combination with inorganic nutrients was added (glucose+N+P treatment) compared to the control (no labile DOM) during the post-pulse phase (Table S3). PE, calculated as the difference in respired willow-derived C in the labile DOM treatments compared to the control, was not statistically significant (Fig. 2, Table S3). Despite this, the average PE was net positive for all treatments in both phases. Incorporation of willow DOM into biofilm biomass at the end of the experiment did also not show any significant differences between the labile DOM treatments and the control (Fig. 4).
Figure 2. Respiration in the microcosms.
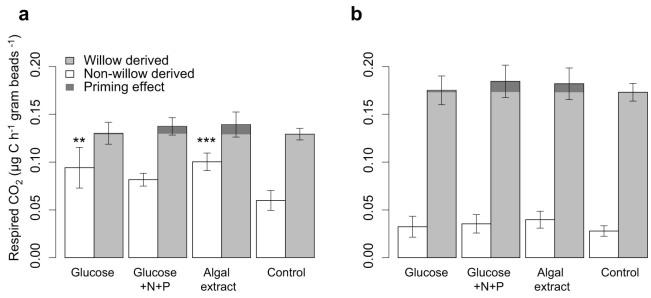
Bars represent respiration of 13C labeled willow (shaded) and non-willow (unshaded: labile DOM, background DOM and biofilm OM) carbon sources during a) the pulse phase and b) during the post-pulse phase. The error bars represent ± 1 standard deviation. The stars over the bars indicate the significance of the difference of the labile DOM treatments compared to the control using one-way ANOVA (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Figure 3. Removal of DOC in the microcosms.
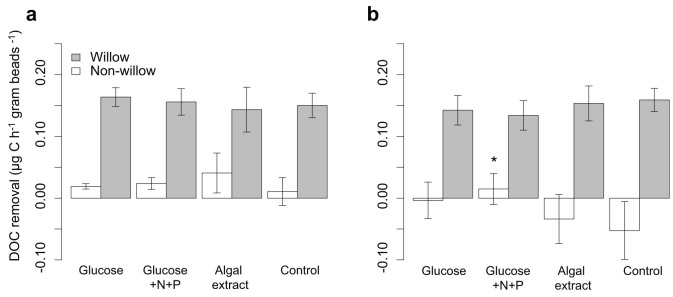
Bars represent removal of 13C labeled, willow DOC (shaded) and non-willow DOC (unshaded: added labile DOM and background DOC) during a) the pulse phase and b) post-pulse phase. Negative removal corresponds to a net release of DOC. The error bars represent ± 1 standard deviation. The stars over the bars indicate the significance of the difference of the labile DOM treatments compared to the control using one-way ANOVA (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Figure 4. The composition of the biofilm biomass in the microcosms.
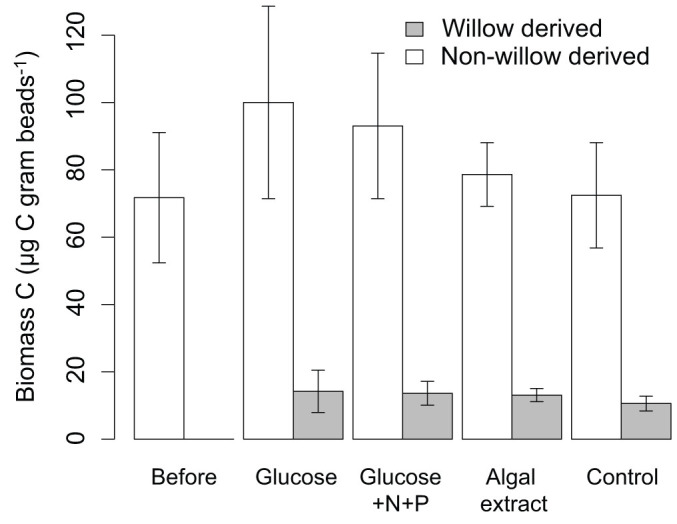
Shaded and unshaded bars represent 13C labeled, willow-derived allochthonous C and non-willow C, respectively. The “before” treatment consists of bioreactors harvested before the start of willow and labile DOM additions. Bioreactors of all other treatments were harvested at the end of the experiment. The error bars represent ± 1 standard deviation.
Metabolism of other (non-willow) carbon pools
Carbon pools other than the willow DOM include the glucose and algal extract additions, natural streamwater DOM, natural particulate organic matter associated with the microcosms and microbial biomass. However, partitioning among these sources was not possible with our methodological approach. The labile DOM additions caused an increase in the respiration of non-willow carbon pools during the pulse phase, which was significant for the glucose and the algal extract treatments (Fig. 2a). This increase likely reflects respiration of the added labile DOM and did not persist in the post-pulse phase (Fig. 2b). There was no significant difference in the removal of non-willow DOC during the pulse phase (Fig. 3a). However, there was a significantly higher removal of non-willow DOC in the glucose+N+P treatment during the post-pulse phase (Fig. 3b).
General operation of the hyporheic microcosms
During the acclimation of the hyporheic zone microbial communities, when microcosms received only stream water, respiration and DOC removal in the microcosms was low with a net removal of streamwater DOC close to zero (Table S2). Supplying the pre-degraded willow DOM during the pulse phase increased the respiration and DOC removal in all microcosms (including the control treatment) indicating that the willow extract still contained DOM fractions that were labile for the hyporheic zone microbial communities (Table S2). Although there were no significant differences in either willow- or non-willow DOC removal (considered separately) during labile DOM additions (Fig. 3a), bulk DOC removal was significantly higher in the glucose and algal extract treatments (Table S2). The removal of inorganic nutrients (NO3 and PO4) was very low or nonexistent throughout the experiment. In the post-pulse phase, there was even a net release of inorganic nutrients from the microcosms (Fig. S3, Table S2), indicating that the hyporheic communities were not limited by these inorganic nutrients during the experiment.
Discussion
In this study, the interactions of allochthonous and autochthonous DOM in the hyporheic zone of streams were addressed, with the aim to uncover if PE is important in these systems. PE could not be detected in this study based on the quantitative analysis of the metabolism of a model, 13C-labeled allochthonous DOM substrate (willow extract DOM) upon the addition of labile DOM in combination with inorganic nutrients.
Studies on the dynamics of allochthonous versus autochthonous carbon pools have a long-standing tradition in aquatic sciences15. However, the concept of PE, which stems from soil science, has only recently crossed the barrier between disciplines to receive attention in the aquatic sciences literature in later years10,11,16,17. Despite the potential importance of PE to carbon cycling, there are still few studies using appropriate methods to detect and quantify PE in aquatic environments, such as isotopic labeling techniques9,18. In a recent study addressing the degradation of soil-derived OM in inland waters19, PE was detected on soil OM in aquatic microcosms supplemented with 13C labeled glucose. However, the PE was small compared to the effect of the aquatic context (soil in water) compared to a terrestrial context (soil only) on the respiration of soil OM19. Franke and colleagues detected both negative and positive PE on natural streamwater DOC when adding 13C-labeled glucose to stream biofilms in bottle incubations20. Negative PE was detected in one stream when glucose was added in combination with inorganic nutrients in summer, while in the same stream in autumn, additions of glucose alone yielded a positive PE corresponding to 40–50% of background streamwater DOC respiration. However, these instances were exceptions in this study as across 4 streams tested, glucose additions typically resulted in no detectable PE.
Other recent aquatic studies have used alternative approaches to address PE. For example, Danger and colleagues observed an increase in the degradation rate of leaf litter in headwater streams in the presence of diatoms, especially under low nutrient conditions, which suggests a positive PE21. Other studies have also reported stimulation of leaf litter degradation processes or organisms involved in litter degradation in the presence of algae22,23. These studies are conceptually similar to ours in the sense that algal exudates are expected to interact with leaf litter OM to yield a PE. However, the degradation of whole leaf litter may differ in several ways to litter-derived DOM, for example in the relative importance of fungal decomposers24. In addition, the lack of quantification of carbon pools using for example stable isotope methods limits what conclusions can be drawn about PE in these studies.
As in any experimental study, there are factors relating to our experimental setup that do not perfectly reflect natural conditions and may therefore compromise the general applicability of our results. For example, natural stream DOM is diverse in composition and origin and presents a challenge to recreate experimentally. Therefore we used a 13C-labeled model substrate (willow DOM) made to resemble allochthonous stream DOM in order to be able to separate metabolism of different carbon pools in our experiment. The willow DOM provides an appropriate representation of leaf litter-derived OM that has undergone some microbial processing, yet may not be representative of the allochthonous DOM most frequently encountered by hyporheic communities. Also, the degree of recalcitrance of the willow DOM is a matter of definition. During the pre-degradation of the willow extract, we observed a dramatic decrease in DOC concentration followed by a leveled off degradation rate (Fig. S1), which was interpreted as an increase in the relative recalcitrance of the solution based on the assumption that labile DOC components are selectively removed, leaving recalcitrant components behind. The term recalcitrance and its use to classify organic matter have raised some controversy in later years (e.g.25 and replies). Indeed, the idea of recalcitrance as a molecular property of organic substances (“intrinsic recalcitrance”) is being challenged, and it has been shown that the persistence of certain organic substances is a function of microbial and environmental controls rather than molecular properties26. Thus, persistence of organic matter is highly dependent on the ecological context and it is therefore not possible to ensure that substrates that resist microbial degradation in one environment will also persist in another environment. Although care was taken to ensure that the willow DOM was produced under similar conditions as our experimental conditions, the relatively high proportion of it that was respired (around 60–70%, also in the control treatment, Table S3) during the experiment indicates that it contained fractions that were indeed labile to microbial heterotrophs in our microcosms. However, we would still expect the willow DOM to be relatively less labile than the glucose and algal extract used to simulate autochthonous DOM inputs.
The inorganic nutrients NO3 and PO4 do not appear to have been limiting in our study, considering the net release of these nutrients during most of the experiment. It has been suggested that PE should occur primarily under inorganic nutrient limiting conditions7,10. This assumption is based on the theory that PE is the result of microbial “nutrient mining” of recalcitrant OM. In summary, recalcitrant OM molecules may contain N and P as well as C, and when more available forms of these inorganic nutrients are not present, microorganisms invest in extracellular enzymes to break down recalcitrant OM, thereby also liberating valuable limiting nutrients. Under non-nutrient limiting conditions, there is no reason for microorganisms to invest in energetically costly recalcitrant OM degradation, and they instead preferentially utilize labile substrates10. The willow DOM had a C:N ratio of >60, making it a poor source of N for nutrient mining organisms. This, in combination with the apparent lack of inorganic nutrient limitation during the experiment may explain the absence of detectable positive PE in our study. The negative PE on stream DOC upon labile carbon and inorganic nutrient additions20 is also in agreement with preferential labile substrate utilization under non-nutrient limiting conditions. However, Carlson and colleagues observed an increased mineralization of marine DOM in microcosms containing Sargasso Sea water when C, N and P were added together, but not when they were added separately27. This suggests a PE that is enhanced, not suppressed, by available inorganic nutrients. In general, aquatic OM may differ substantially from soil OM with respect to C:N:P ratios. Soil organic matter typically has a C:N ratio <2028, making it a potentially good source of N for N-mining microorganisms, while aquatic OM pools tend to be more C rich in general29,30. In this case, microbial nutrient mining of recalcitrant OM may have limited relevance as an underlying mechanism of aquatic PE.
We also observed a net release of DOM originating from non-willow carbon pools during the post pulse phase of the experiment (Fig. 3). In addition, the net-zero removal of DOC during the streamwater phase in combination with a positive respiration rate (Table S2) indicates that biofilms were simultaneously removing and releasing DOC. These observations may be a sign that the biofilm community was in a state of turnover, with senescent cells contributing to release of DOC. This finding contrasts with earlier studies using similar bioreactor microcosm approaches, where a net release of DOC has not been observed31, presumably because the biofilms had been established over longer time periods and their microbial communities were in a near steady state. However, biofilms are likely to also be in a state of turnover in natural streams, where storm events and other environmental changes continuously disturb sediments in the hyporheic zone. Therefore, our results are relevant when considering certain dynamic conditions of natural streams, but may not apply for other situations.
Heterotrophic stream biofilms are predominantly perceived as sinks of DOM and inorganic nutrients. While it is obvious that their contribution to solute budgets is a result of both removal and release, due to a net removal the release is seldom viewed as significant or important. However, re-working of DOM by microbial heterotrophs may lead to increased recalcitrance, a process which has been referred to as the “microbial carbon pump” in marine waters32. Perhaps the truly persistent DOM in inland waters is mainly the product of microbial metabolism of indigenous heterotrophic communities, and not the left-over “recalcitrant” components of terrestrial primary production.
Although PE was not detected in this study using the current methodological approach, it is too early to say if PE is unimportant in streams, or other aquatic environments. Stream biofilms may still mediate PE under different conditions than addressed by our experiment, such as under nutrient limitation20,21. Also, the hyporheic zone, which was investigated in this study, may not be an ideal site for PE to occur, due to the potentially low amounts of labile DOM from primary producers penetrating stream sediments. Benthic biofilms, in which algae and heterotrophic bacteria coexist in close proximity, may instead be the “hotspots” of PE in stream ecosystems. In general, more studies that quantitatively assess PE in aquatic ecosystems using stable isotope-based methods are needed to clarify whether changes in various aspects of microbial carbon processing upon labile additions, as reported in some studies21,22,23, actually relate to PEs that are significant in the ecosystems where they may occur. In addition, when PE does occur, it is important to uncover under what conditions, and what the underlying mechanisms are to assess the impacts that it may have in a changing environment.
Methods
A setup including 25 microcosms, in the form of plug-flow bioreactors31, was employed to study metabolism of DOM by stream biofilms. The microcosms were packed with glass beads inoculated with natural steam biofilm communities from the stream Oberer Seebach, in Lunz am See, Austria. They were fed with oligotrophic stream water for 16.5 days to acclimatize to laboratory conditions. Five of the microcosms were harvested before the start of experimental DOM amendments to sample the original biofilm biomass. All remaining microcosms were amended with allochthonous DOM in the form of a cold-water extract of 13C labeled leaves and stems of crack willow (Salix fragilis) that had been subjected to pre-degradation by stream biofilm communities to remove the most labile organic components (see supplementary methods and Fig. S1). In addition to willow DOM, 15 of the microcosms were amended with different labile DOM amendments simulating autochthonous inputs to the hyporheic zone, while 5 microcosms only receiving willow DOM served as control.
The labile DOM treatments were: Glucose (0.44 mg L−1), Glucose+N+P (0.44 mg L−1 glucose, 2200 μg L−1 N-NO3, 6.8 μg L−1 P-PO4) and an algal extract (0.44 mg L−1 algal DOC). The concentration of willow DOM was 0.88 mg L−1 DOC. All DOM additions were mixed with stream water that had an average background DOC concentration of 1.09 mg L−1. All target concentrations were within the range of naturally occurring DOC and inorganic nutrient concentrations in this stream. See Table S1 for detailed properties of the different additions and media.
The experiment was divided into two main phases; first, the labile DOM amendments were carried out continuously over 7 days in the treated microcosms (pulse phase). This phase simulated a pulsed input of autochthonous DOM, such as during an algal bloom. All microcosms were simultaneously receiving willow DOM simulating a background of allochthonous DOM inputs. The pulse phase was followed by willow DOM amendments alone for 14 days (post-pulse phase) at the same willow DOM concentration as during the pulse phase. See Fig. S2 for a detailed illustration of additions and phases.
Liquid samples for measurements of dissolved oxygen, the concentration and stable isotope composition of DOC and DIC, as well as inorganic nutrient concentrations were taken at 13 times during the experiment. The inflowing and outflowing liquid of the hyporheic microcosms was sampled, allowing calculation of removal rates which could be used to estimate accumulated removal for each parameter for the two phases of the experiment according to:
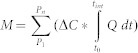 |
Where M is the accumulated mass of C, N or P removed or released by a microcosm during the pulse- or post-pulse phase, P1 and Pn refer to the first and last sampling points in one phase, ΔC is the difference in concentration of C (DOC or respired CO2), N or P between input and output of the microcosm measured at one sampling point, Q is the flowrate, and t0 and tint represent the duration of a representative time interval encompassing one sampling point. The fluxes in each phase were finally normalized to the mass of beads in each microcosm (a proxy for biomass) and expressed as an hourly rate to allow comparison between the phases. Respired CO2 was calculated from oxygen removal using an empirically derived respiratory coefficient (see supplementary methods).
Biomass for determination of C and N content and stable isotope ratios (12C:13C) was sampled before the start of the experiment and at the end of the experiment, when all microcosms were destructively sampled.
To partition the removal, respiration and biomass accumulation of willow DOM from other carbon sources (labile DOM amendments, background stream and biofilm C) using the stable isotope ratios of DOC, DOC and biomass, a two end member linear mixing model was employed33.
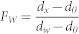 |
Where FW is the fraction of willow C and other C in the mixture, dX is the measured isotopic signature of the C pool of interest (respired CO2, DOC or biomass), and dW and do are the isotopic signatures of the willow DOM (9.35 atom % 13C) and non-willow C (natural abundance, 1.08 atom % 13C), respectively. PE was calculated as the surplus willow-derived respiration in the labile DOM treatments compared to the mean willow-derived respiration of the control treatment.
Significant differences between treatments were tested for using one-way anova, followed by Tukey's honest significant differences. All calculations and statistics were carried out in R34.
Author Contributions
T.J.B. conceived the study. The experiment was designed and planned by M.M.B., K.W., T.J.B. with contributions from W.W. and L.A.K. Experimental work was carried out by M.M.B., K.W., N.R.B. and E.R.H. Data analysis and writing was carried out by M.M.B. All authors contributed to creative discussions and editing of the final versions of the manuscript.
Supplementary Material
Supplementary material
Acknowledgments
The authors wish to thank G. Singer and C. Preiler for advice on microcosm setup, S. Hengsberger and M. Winkler for technical assistance during the experiment, M. Watzka, G. Steniczka, C. Hinterleitner and B. Wild for technical assistance during sample processing and W. R. Hunter for help with data analysis. Financial support came from the Austrian Science Fund (FWF) to T.J.B. (START Y420-B17 and P23420) and from a Marie Curie Individual Fellowship (PIEF-GA-2010-274895) to M.M.B.
References
- Mulholland P. J., Marzolf E. R., Webster J. R., Hart D. R. & Hendricks S. P. Evidence that hyporheic zones increase heterotrophic metabolism and phosphorus uptake in forest streams. Limnol Oceanogr 42, 443–451 (1997). [Google Scholar]
- Battin T. J., Kaplan L. A., Newbold J. D. & Hendricks S. P. A mixing model analysis of stream solute dynamics and the contribution of a hyporheic zone to ecosystem function. Freshwater Biology 48, 995–1014 (2003). [Google Scholar]
- Jones J. B. & Mulholland P. J. Streams and Ground Waters. (Academic Press, 1999). [Google Scholar]
- Dahm C. N. Pathways and mechanisms for removal of dissolved organic carbon from leaf leachate in streams. Can J Fish Aquat Sci 38, 68–76 (1981). [Google Scholar]
- Seitzinger S. et al. Molecular-level chemical characterization and bioavailability of dissolved organic matter in stream water using electrospray-ionization mass spectrometry. Limnol Oceanogr 50, 1–12 (2005). [Google Scholar]
- Singer G., Besemer K., Scmitt-Kopplin P., Hödl I. & Battin T. J. Physical heterogeneity increases biofilm resource use and its molecular diversity in stream mesocosms. PLoS ONE 1–11 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzyakov Y. Priming effects: Interactions between living and dead organic matter. Soil Biol Biochem 42, 1363–1371 (2010). [Google Scholar]
- Bingeman C. W., Varner J. E. & Martin W. P. The effect of the addition of organic materials on the decomposition of an organic soil. Soil Sci Soc Am J 17, 34–38 (1953). [Google Scholar]
- Blagodatskaya E. & Kuzyakov Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fert Soils 45, 115–131 (2008). [Google Scholar]
- Guenet B., Danger M., Abbadie L. & Lacroix G. Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecology 1–39 (2010). [DOI] [PubMed] [Google Scholar]
- Bianchi T. S. The role of terrestrially derived organic carbon in the coastal ocean: A changing paradigm and the priming effect. PNAS 108, 19473–19481 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufdenkampe A. K. et al. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front Ecol Environ 9, 53–60 (2011). [Google Scholar]
- Hoellein T. J., Bruesewitz D. A. & Richardson D. C. Revisiting Odum (1956): A synthesis of aquatic ecosystem metabolism. Limnol Oceanogr 58, 2089–2100 (2013). [Google Scholar]
- Battin T. J. et al. Biophysical controls on organic carbon fluxes in fluvial networks. Nat Geosci 1, 95–100 (2008). [Google Scholar]
- Findlay S. E. G. & Sinsabaugh R. L. Aquatic Ecosystems. (Academic Press, 2003). [Google Scholar]
- van Nugteren P. et al. Seafloor ecosystem functioning: the importance of organic matter priming. Mar Biol 156, 2277–2287 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C. S., Lyon D. R. & Ziegler S. E. Cycling of two carbon substrates of contrasting lability by heterotrophic biofilms across a nutrient gradient of headwater streams. Aquat Sci 75, 235–250 (2013). [Google Scholar]
- Kuzyakov Y., Friedel J. & Stahr K. Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32, 1485–1498 (2000). [Google Scholar]
- Guenet B. et al. Fast mineralization of land-born C in inland waters: first experimental evidences of aquatic priming effect. Hydrobiologia 721, 35–44 (2013). [Google Scholar]
- Franke D., Bonnell E. J. & Ziegler S. E. Mineralisation of dissolved organic matter by heterotrophic stream biofilm communities in a large boreal catchment. Freshwater Biol 58, 2007–2026 (2013). [Google Scholar]
- Danger M., Cornut J., Chauvet E., Chavez P. & Elger A. Benthic algae stimulate leaf litter decomposition in detritus-based headwater streams: a case of aquatic priming effect? Ecology 94, 1604–1613 (2013). [DOI] [PubMed] [Google Scholar]
- Kuehn K. A., Francoeur S. N., Findlay R. H. & Neely R. K. Priming in the microbial landscape: periphytic algal stimulation of litter-associated microbial decomposers. Ecology (2013). [DOI] [PubMed] [Google Scholar]
- Rier S. T., Kuehn K. A. & Francoeur S. N. Algal regulation of extracellular enzyme activity in stream microbial communities associated with inert substrata and detritus. J N Am Benthol Soc (2007). [Google Scholar]
- Gessner M. O. & Chauvet E. Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75, 1807–1817 (1994). [Google Scholar]
- Kleber M. What is recalcitrant soil organic matter? Environ Chem 7, 320–332 (2010). [Google Scholar]
- Schmidt M. W. I. et al. Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56 (2011). [DOI] [PubMed] [Google Scholar]
- Carlson C. et al. Effect of nutrient amendments on bacterioplankton production, community structure, and DOC utilization in the northwestern Sargasso Sea. Aquat Microb Ecol 30, 19–36 (2002). [Google Scholar]
- Batjes N. H. Total carbon and nitrogen in the soils of the world. Eur J Soil Sci 47, 151–163 (1996). [Google Scholar]
- Hopkinson C. S. & Vallino J. J. Efficient export of carbon to the deep ocean through dissolved organic matter. Nature 433, 142–145 (2005). [DOI] [PubMed] [Google Scholar]
- Grimm N. B. et al. Merging aquatic and terrestrial perspectives of nutrient biogeochemistry. Oecologia 137, 485–501 (2003). [DOI] [PubMed] [Google Scholar]
- Kaplan L. A. & Newbold J. D. Measurement of streamwater biodegradable dissolved organic carbon with a plug-flow bioreactor. Water Res 29, 2696–2706 (1995). [Google Scholar]
- Jiao N. et al. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nat Rev Microbiol 8, 593–599 (2010). [DOI] [PubMed] [Google Scholar]
- Fry B. & Sherr E. B. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib Mar Sci 27, 13–47 (1984). [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material