Figure 1.
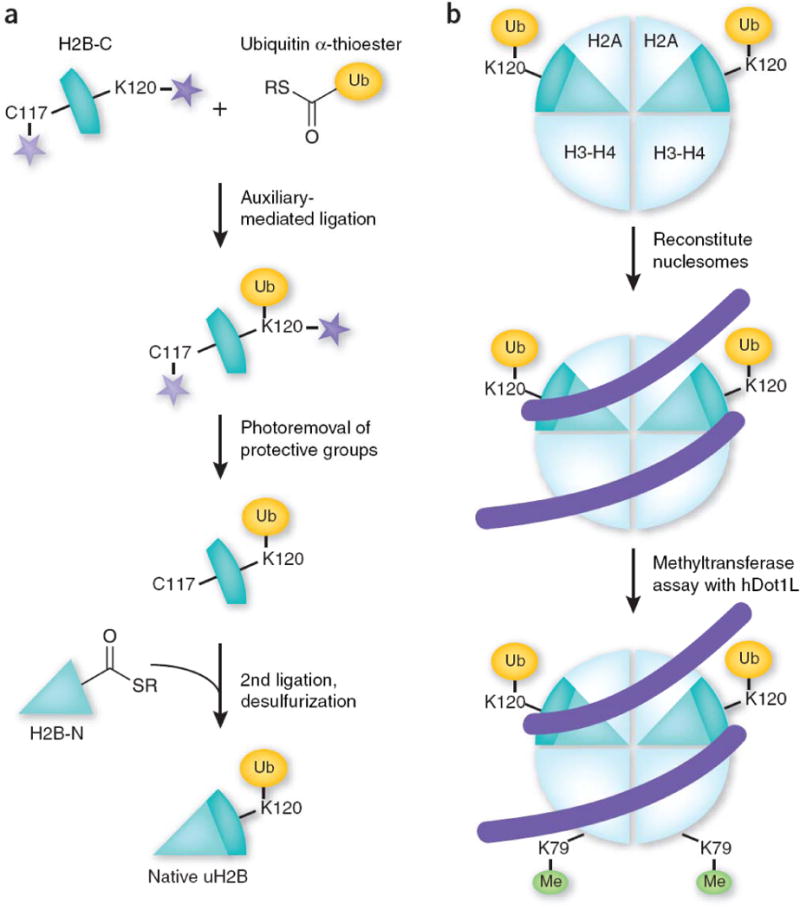
Making of synthetic uH2B for hDot1L-mediated methyltransferase assay. (a) Strategy for making native uH2B using a double native ligation approach. A C-terminal H2B peptide encompassing residues 117–125 and containing a cysteine substitution for Ala117 (H2B-C) is ligated to ubiquitin α-thioester. After photoremoval of the protective group and the ligation auxiliary (stars), the intermediate is ligated to the N-terminal part of H2B (residues 1–116) bearing a C-terminal α-thioester. Raney nickel desulfurization is then performed to convert the cysteine at position 117 back to alanine, which results in the formation of native uH2B. (b) uH2B molecules are incorporated into nucleosomes and subsequently subjected to a histone methyltransferase assay using the H3K79 methyltransferase hDot1L.
