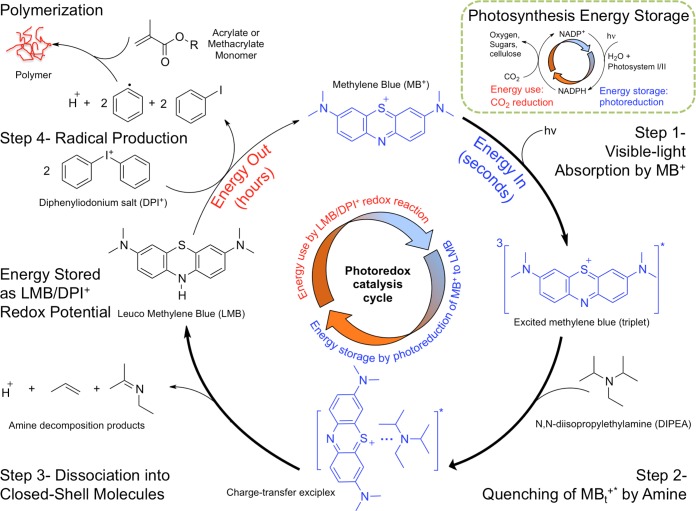
Figure 2.
Free radical-initiated polymer synthesis with light energy harvesting cycle. Step 1: Visible-light (hv) excitation of MB+ to the singlet state (not shown), which quickly decays to the longer-lived triplet state (MBt+*) via intersystem crossing. Step 2: Excess DIPEA quenches MBt+* to colorless LMB via transfer of two electrons and one proton (reaction 1) through formation of a charge-transfer excited-state complex (exciplex). Step 3: After a 2e–/1H+ transfer, the exciplex separates into LMB and DIPEA-decomposition products. DIPEA decomposes to closed-shell molecules and does not initiate polymerization. Step 4: LMB is oxidized back to MB+ by DPI+ to produce two phenyl radicals per LMB. Phenyl radicals are responsible for the fast initiation of chain-growth polymerization of HEMA. Faster (thicker arrows) MB+ reduction and slower (thinner arrows) reoxidation steps allow LMB to accumulate and also create a lag time between light absorption and radical generation. Thus, energy is stored as an electrochemical potential between LMB and DPI+, which produces radicals beyond light absorption. This is analogous to the NADP+/NADPH cycle (inset) known in photosynthesis in which the transfer of 2e–/1H+ in the photoredox cycle stores light energy in the form of a chemical potential that is used to reduce carbon dioxide to higher molecular weight sugars and carbohydrates.