Figure 1.
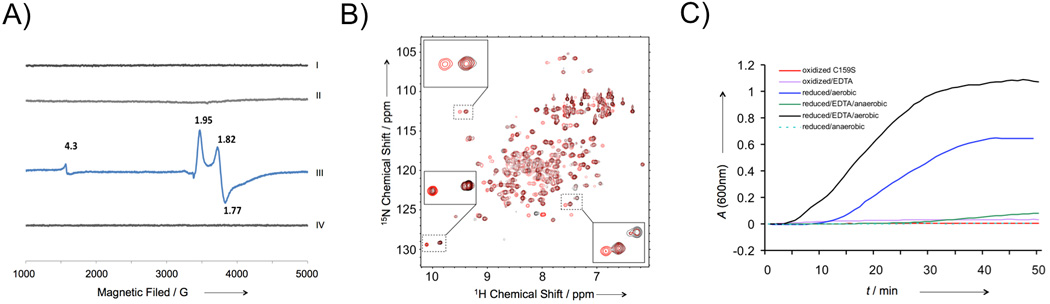
Characterization of the FBXL5 hemerythrin-like domain. A) EPR spectra of oxidized (I), aerobically purified (II), partially reduced (III), and fully reduced (IV) FBXL5 Hr. B) 1H-15N HSQC NMR spectra of FBXL5 Hr purified under aerobic conditions (red) and the reduced protein under anaerobic conditions (black). The insets show the cross-peaks that correspond to two different conformations. C) Stability of FBXL5 Hr under different conditions. The denaturation and aggregation of FBXL5 Hr was monitored spectrophotometrically at 600 nm. The concentration of the samples used is 40 µM.
