Figure 4.
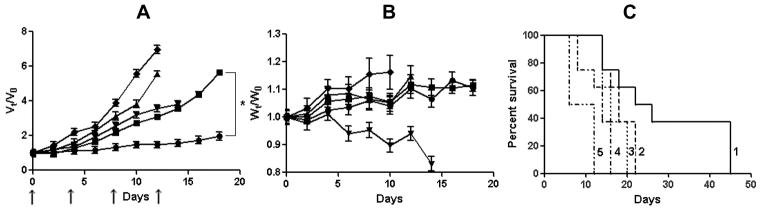
In vivo antitumor efficacy of (CDDP+PTX)/cl-micelles in A2780 human ovarian cancer xenograft-bearing female nude mice. Relative changes in (A) tumor volume and (B) body weight were measured following intravenous administration of (CDDP+PTX)/cl-micelles(●) or CDDP/cl-micelles (■) or PTX/cl-micelles (▲) or free CDDP (▼) or 5% dextrose (◆). Drug formulations were injected in100 μL at a dose of 4 mg CDDP or 1mg PTX equivalents/kg body weight 4 times at 4-day intervals as indicated by the arrows. Values indicated are means ± SEM (n = 8). (C) Kaplan–Meier analysis of overall survival in (CDDP+PTX)/cl-micelles group (1) or CDDP/cl-micelles group (2) or PTX/cl-micelles group (4) or free CDDP group (3) or control group (5). Tumor volume and body weight are normalized with respect to tumor volume or body weight at day 0. * P<0.05.
