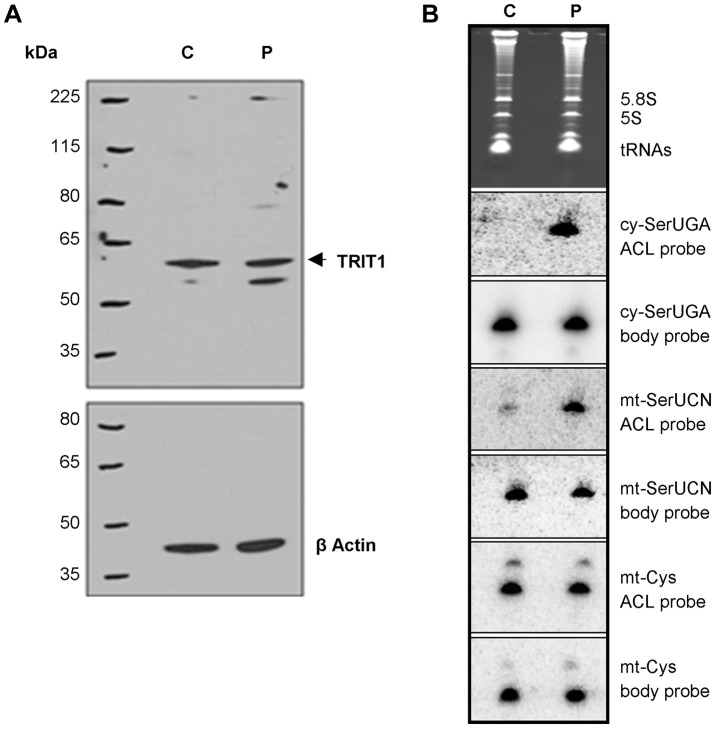
Figure 4. The TRIT1 mutation disrupts modification activity on cytosolic and mitochondrial tRNAs but not enzyme abundance.
A) No decrease in the levels of the native TRIT1 protein in patient fibroblasts was observed by immunoblotting (using β-actin as a loading control) B) The isopentenyl modification status of both mitochondrial (mt-) and cytosolic (cy-) tRNAs in patient fibroblasts (lane P) compared to controls (lane C); by this approach a positive signal is due to lack of isopentenyl modification as detected by an anticodon loop (ACL) probe (the bulky modification on the N of adenine blocks base pairing with the probe, such that no signal for cy-tRNASer(UGA) with the ACL probe indicates efficient modification in the control cells [11]); a body probe to a different region of the same tRNA is used as a control for calibration and calculation of steady-state levels. Each panel shows hybridisation of the same blot with a different probe as indicated to the right. The cytosolic tRNASer(UGA) is poorly modified in patient fibroblasts (strong ACL probe signal), but tRNASer(UGA) steady-state levels are unchanged. Mt-tRNASer(UCN) is also poorly modified in patient fibroblasts, although a small pool of mt-tRNASer(UCN) in control fibroblasts lacks the modification. The modification appears to be influential on mt-tRNASer(UCN) stability, as steady-state levels are decreased by 40% in the patient. The non-substrate mt-tRNACys was probed as a control.