Abstract
AIM: To evaluate the clinical significance of cystatin C and renal resistive index for the determination of renal function in patients with liver cirrhosis.
METHODS: We conducted a study of 63 patients with liver cirrhosis. A control group comprised of 30 age and gender-matched healthy persons. Serum cystatin C was determined in all study subjects and renal Doppler ultrasonography was made. Estimated glomerular filtration rate from serum creatinine (GFRCr) and cystatin C (GFRCys) was calculated.
RESULTS: We confirmed significant differences in values of cystatin C between patients with different stages of liver cirrhosis according to Child-Pugh (P = 0.01), and a significant correlation with model of end stage liver disease (MELD) score (rs = 0.527, P < 0.001). More patients with decreased glomerular filtration rate were identified based on GFRCys than on GFRCr (P < 0.001). Significantly higher renal resistive index was noted in Child-Pugh C than in A (P < 0.001) and B stage (P = 0.001). Also, a significant correlation between renal resistive index and MELD score was observed (rs = 0.607, P < 0.001). Renal resistive index correlated significantly with cystatin C (rs = 0.283, P = 0.028) and showed a negative correlation with GFRCys (rs = -0.31, P = 0.016).
CONCLUSION: Cystatin C may be a more reliable marker for assessment of liver insufficiency. Additionally, cystatin C and renal resistive index represent sensitive indicators of renal dysfunction in patients with liver cirrhosis.
Keywords: Liver cirrhosis, Cystatin C, Renal resistive index
Core tip: Early diagnosis of renal dysfunction is important to prevent serious complications. We conducted a study of 63 patients with liver cirrhosis and 30 healthy controls. Serum cystatin C (CysC) was measured and renal Doppler ultrasonography was performed. More patients with decreased glomerular filtration rate (GFR) were identified based on CysC GFR than on creatinine GFR. Higher renal resistive index in advanced disease by Child-Pugh and model of end stage liver disease was noticed. Renal resistive index (RRI) correlated with CysC and negatively correlated with glomerular filtration rate from serum cystatin C. Cystatin C may be a more reliable marker for liver insufficiency assessment. CysC and RRI represent sensitive indicators of renal dysfunction in liver cirrhosis.
INTRODUCTION
Cirrhosis of the liver is often accompanied by functional renal failure particularly in advanced stages of liver disease. Hemodynamic alterations with reduced effective arterial blood volume and peripheral vasodilation are followed by activation of vasoconstrictive hormones (renin-aldosterone, vasopresin, endothelin) and neurohumoral systems (including increased activity of nervous system)[1,2]. The most common functional renal abnormalities in patients with cirrhosis are an impaired ability to excrete sodium and water and a reduction of renal blood flow and glomerular filtration rate, the latter two being secondary to vasoconstriction of the renal circulation[3]. Hence renal failure is directly linked to the mortality rate of cirrhotic patients, it is of a great clinical importance to monitor renal function closely in order to estimate the prognosis and determine the optimal therapeutic option[4].
Whereas patients with a significantly impaired glomerular filtration rate can be diagnosed easily by elevated serum creatinine (Cr) concentrations, moderately reduced renal function may go unnoticed by this conventional parameter. Nevertheless, the protease inhibitor cystatin C (CysC) has been proposed as a specific marker of glomerular filtration rate (GFR) and an early indicator of impaired renal function[5].
CysC is a non-glycosylated 13 kDa protein, produced at a constant rate by all nucleated cells, freely filtered by the glomeruli and subsequently metabolized in the proximal tubules[6]. Opposed to Cr, CysC is independent of gender, age, and muscle mass and not influenced by serum bilirubin, inflammation, or malignancy[7,8].
The aim of the study was to evaluate the clinical significance of CysC and renal blood flow for the determination of renal function in patients with liver cirrhosis.
MATERIALS AND METHODS
Subjects
We conducted a study of 63 patients, aged 18 years and above, with alcoholic or viral liver cirrhosis examined and treated between December 2011 and September 2013 at the Clinic for Gastroenterology and Hepatology, Clinical Center of Serbia, Belgrade. A healthy control group comprised of 30 age and gender-matched subjects. Diagnostic approach was based on clinical clues from the patient’s medical history (e.g., consumption of pure alcohol more than 50 g/d over a five-year period), physical examination, laboratory tests, abdominal ultrasonography and upper endoscopy; liver biopsy was performed in 15 (23.8%) patients. Laboratory analyses included tests of hepatocyte integrity, cholestasis, synthetic liver function tests and etiological tests.
The degree of liver insufficiency was assessed according to the Child-Pugh classification and divided into three stages: A, B and C (score A ≤ 6, B 7-9, and C ≥ 10)[9]. The diagnosis of hepatic encephalopathy was based on clinical criteria, and the severity of hepatic encephalopathy was based on the West Haven Criteria for grading of mental status[10]. The model of end stage liver disease (MELD) has also been used to assess patients with liver cirrhosis[11].
All respondents were evaluated for any superimposed conditions such as infection, intrinsic renal disease, chronic obstructive pulmonary disease, congestive heart failure, thyroid dysfunction, and diabetes mellitus. The following exclusion criteria were applied: patients with hepatocellular carcinoma, gastrointestinal bleeding, or hepatorenal syndrome (HRS). Patients receiving corticosteroids, antiviral agents, angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors, amynoglicosides, nonsteroidal anti-inflamatory drugs, or aminoacids L-arginine and L- ornithine were also excluded from the study.
Biochemistry
Diuretics were stopped in all patients, at least 24 h before laboratory testing. Patients were adviced to adopt a low-sodium diet (less than 40 mmol/d). Serum samples were obtained on the day of urine collection. Venous blood samples were collected in vacutainers without additives, centrifuged at 3500 rpm (about 2000 g) and preserved at -80 °C after separation. CysC serum concentration was determined by the PENIA method (Particle-Enhancesd Nephelometric Immuno-Assay), using the SIEMENS (Marburg, Germany) tests, on a laser nephelometer (BN IIDadeBehring). CysC referent value was 0.59-1.04 mg/L. Cr was determined according to the kinetic Jaffe’s method, using an automated biochemical analyzer (Olympus AU 400) and commercially available assay kits by the same manufacturer. Cr referent value for men was 59-104 μmol/L and for women 45-84 μmol/L. Creatinine clearance (CLCr) was calculated as a product of urinary Cr and 24-h urine volumen divided by serum Cr (μmol/L) and multiplied by 1440. Referent values for 24-h urinary creatinine excretion were 23 mg/kg ideal body weight for men and of 17 mg/kg ideal body weight for women[12].
Estimated GFR was calculated from serum Cr using the Modification of Diet in Renal Disease (MDRD) equation: eGFR = 186 × sCr-1.154 × age-0.203 × 1.212 (if African American) × 0.742 (if female)[13], and from serum CysC using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation: eGFR = 127.7 × CysC-1.17 × age-0.13 × 0.91 (if female) × 1.06 (if African American)[14]. Serum sodium (sNa+) referent value was 135-148 mmol/L, and urine sodium (uNa+) referent value for men was 40-220 mmol/L and for women 27-287 mmol/L.
Abdominal and renal Doppler ultrasonography
Ultrasonography (Toshiba Core Vision, with Doppler duplex convex probe, 3.5 MHz) was performed to examine the liver size, echo structure of the hepatic parenchyma and possible focal changes, spleen diameter, and presence of ascites. Renal color Doppler duplex ultrasonography was used to evaluate renal resistive index (RRI). The renal arteries were evaluated bilaterally of the distal arcuate branches. RRI equals peak systolic velocity minus the final diastolic velocity divided by the peak systolic velocity. RRI less than 0.7 is considered normal and was calculated based on the mean value of renal arteries[15,16].
Statistical analysis
One-sample Kolmogorov-Smirnov and Shapiro-Wilk tests were performed to determine whether the data showed normal distribution. t test or Mann-Whitney test was applied to assess the differences in investigated parameters. Analysis of variance (ANOVA) or Kruskal-Wallis test was applied to assess the influence of the investigated parameters. After assessing overall effects of a factor by means of ANOVA, post-hoc multiple comparison procedures with Bonferonni correction were performed to determine individual differences between the groups. Pearson’s (r) or Spearman’s correlation (rs) procedures were performed to evaluate the relationship between different variables. McNemar’s test was used to assess the differences in detecting renal function using two different approaches. The Statistical Package for Social Sciences version 15 (SPSS Inc., Chicago, IL, United States) was used for statistical analyses, at the 0.05 level of significance.
Ethical considerations
The study was conducted in accordance with Guidelines for Good Clinical Practice, the Declaration of Helsinki, and local laws and regulations. The protocol was approved by joint Research and Ethics Committee of the Clinical Center of Serbia, Belgrade, filed under number 2385/5. Written informed consent was obtained from all the participants in the study.
RESULTS
The patient group comprised 47 (74.6%) males and 16 (25.4%) females. The average age of the patients was 50.8 ± 13.5. Alcoholic cirrhosis was diagnosed in 41 (65.1%) and viral cirrhosis in 22 (34.9%) patients.
The average value of CysC measured in patients with liver cirrhosis was 1.09 ± 0.42 mg/L, while it was 0.88 ± 0.12 mg/L in the control group. Comparing these groups, we have confirmed significantly higher CysC in patients with cirrhosis (P = 0.036). Increased values of CysC were observed in 23 (40%) patients.
The liver insufficiency degree, determined by generally accepted Child-Pugh classification, was divided into three stages: A in 23 (36.5%), B in 21 (33.3%) and C in 19 (30.2%) patients. Patients’characteristics based on Child-Pugh score are presented in Table 1. MELD score ranged from 8 to 26.
Table 1.
Characteristics of enrolled patients based on Child Pugh score (n = 63)
| Characteristic | A | B | C | P value |
| Bilirubin (μmol/L) | 17.7 | 33.5 | 55.1 | < 0.001 |
| AST (U/L) | 34 | 39 | 57 | 0.035 |
| ALT (U/L) | 32 | 29 | 40 | 0.187 |
| ALP (U/L) | 112 | 109 | 113 | 0.888 |
| GGT (U/L) | 103 | 73 | 51 | 0.153 |
| Albumin (g/L) | 36 | 32 | 25 | < 0.001 |
| sNa+ (mmol/L) | 139 | 137 | 135 | 0.008 |
| Cr (μmol/L) | 67 | 70 | 74 | 0.540 |
| CLCr (mL/min) | 120.7 | 114 | 104 | 0.823 |
| GFRCr (mL/min per 1.73 m2) | 129.9 | 116.1 | 111 | 0.476 |
| CysC (mg/L) | 0.83 | 1.09 | 1.12 | 0.010 |
| GFRCys(mL/min per 1.73 m2) | 93.3 | 67.1 | 64.7 | 0.017 |
| RRI | 0.63 | 0.7 | 0.75 | < 0.001 |
All data are presented as median values. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; GGT: Gamma-glutamyl transferase; sNa+: Serum sodium; Cr: Creatinine; CLCr: Creatinine clearance; GFRCr: Glomerular filtration rate based on creatinine; CysC: Cystatin C; GFRCys: Glomerular filtration rate based on cystatin C; RRI: Renal resistive index.
Post-hoc comparisons showed statistically significant differences in values of CysC between Child-Pugh A and B (P = 0.014) and between A and C (P = 0.007) stages, while there was no difference between B and C stages (P > 0.05) (Table 1). Moreover, we confirmed a statistically significant correlation between CysC and MELD score (Figure 1).
Figure 1.
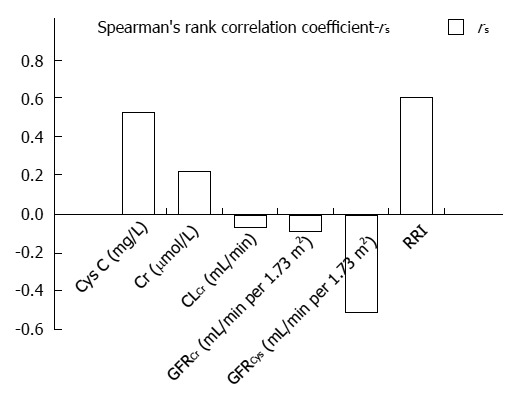
Correlation of renal parameters with model of end stage liver disease score. rs: Spearman’s rank correlation coefficient; CysC: Cystatin C (P < 0.001); Cr: Creatinine (P = 0.091); CLCr: Creatinine clearance (P = 0.557); GFRCr: Glomerular filtration rate based on creatinine (P = 0.460); GFRCys: Glomerular filtration rate based on cystatin C (P < 0.001); RRI: Renal resistive index (P < 0.001).
Increased Cr values were detected in 7 (11.1%) patients, and all of them had increased values of CysC. Mean values for 24-h urinary creatinine excretion for men was 18.3 mg/kg, and for women 16.3 mg/kg. Decreased creatinine values were detected in 32/47 (68%) men and 9/16 (56.3%) women. There were no significant differences in Cr values between the stages of liver cirrhosis in regards to Child-Pugh classification. Furthermore, we detected no significant correlation between Cr and MELD score (Figure 1).
Decreased CLCr was detected in 24 (38%) patients with an average value of 57.2 ± 21.2 mL/min. Reduced CLCr in 9 out of 23 (39.1%) patients with Child-Pugh A, 8 out of 21 (38.1%) with B and 7 out of 19 (36.8%) with stage C were confirmed. No correlation was observed between CLCr and MELD score (Figure 1). Also we detected no statistically significant differences in CLCr between Child-Pugh stages. Additionally, CLCr negatively correlated with CysC and Cr (rs = -0.415, P = 0.001; rs = -0.511, P < 0.001, respectively).
There was a strong negative correlation between increased CysC and decreased sNa+ in patients (r = -0.9, P = 0.002). In contrast, increased Cr values did not show a significant correlation with sNa+ concentration (P > 0.05). In patients with decreased urinary excretion of sodium, CysC correlated negatively with uNa+ concentration (r = -0.748, P = 0.05), while Cr showed no significant relation with uNa+ (P > 0.05).
The mean GFR estimated using Cr (GFRCr) was 113.5 mL/min per 1.73 m2. Lower values of GFRCr (< 90 mL/min per 1.73 m2) were observed in 13 (20.6%) patients, and there was no statistically significant difference between Child-Pugh stages. Lower values of GFRCr (< 60 mL/min per 1.73 m2) were observed in 7 (11.1%) patients (P > 0.05). No significant correlation was observed in GFRCr between Child-Pugh stages (Table 1). Moreover, no significant correlation was detected between GFRCr and MELD score (Figure 1).
Mean GFR based on CysC (GFRCys) was 77.6 mL/min per 1.73 m2. Lower values of GFRCys < 90 mL/min per 1.73 m2 were observed in 40 (63.5%) patients and GFRCys < 60 mL/min per 1.73 m2 in 16 (25.4%) patients, with no statistically significant difference between Child-Pugh stages (P > 0.05).
Mean GFRCys in patients with normal values of CysC was significantly higher than that in patients with increased values of CysC (94.8 ± 15.0 mL/min per 1.73 m2 vs 53.5 ± 14.5 mL/min per 1.73 m2, P < 0.001, respectively). Statistically significant differences in GFRCys between Child-Pugh stages were observed (Table 1). Post-hoc comparisons showed differences in GFRCys between Child-Pugh stages A and B (P = 0.006) and between A and C (P = 0.005). Also, a significant correlation between GFRCys and MELD was determined (Figure 1). A moderate degree of correlation was found between GFRCys and GFRCr in patients with liver cirrhosis (rs = 0.64, P < 0.001). We identified significantly more patients with decreased GFR based on CysC than on Cr (P < 0.001).
We noticed that RRI was significantly higher in cirrhotic patients than in controls (P = 0.005). RRI was already more increased in 27 (43%) patients with ascites when compared to 36 (57%) without ascites (P = 0.005).
Also, RRI was significantly influenced by Child-Pugh stage (Table 1). Comparisons showed markedly higher RRI in Child-Pugh stage C than in A (P < 0.001) and B stage (P = 0.001). Also, a significant correlation was noted between RRI and MELD (Figure 1).
RRI correlated significantly with CysC (rs = 0.283, P = 0.028) and showed a significant negative correlation with GFRCys (rs = -0.31, P = 0.016). However, we detected no relationship between RRI and Cr, CLCr, or GFRCr.
DISCUSSION
Early diagnosis of impaired renal function, particularly decreased GFR, is very important to prevent serious complications[5]. The gold standard for determining GFR is to measure the clearance of an exogenous substances such as chromium-51 labeled ethylenediamine tetraacetic acid (51Cr-EDTA) and inulin. Procedures determining GFR using exogenous substances are invasive and carry a risk for patients, usually are considered too expensive and time consuming for routine clinical use[17]. Moreover, procedure for measuring inulin clearance is impractical because of the necessity for steady-state infusion, a urine bladder catheter, and possible interference from blood glucose[18].
The endogenous marker of GFR most commonly used in routine clinical and laboratory practice is serum Cr. However, in liver cirrhosis, specific non-renal factors may influence Cr concentration. Protein-calorie malnutrition, muscle wasting, and impaired liver function will directly reduce Cr production[19]. Moreover, ascites and peripheral edema can also decrease the Cr due to larger area for distribution[20,21]. We report that reduced urinary creatinine excretion in cirrhosis correlates with anthropometrically estimated muscle mass and is not related to reduced liver function. Our results are consistent with a previous study conducted by Pirlich et al[22]. Given the fact that serum Cr systematically overestimates renal function, mild degree of renal insufficiency may go unnoticed as Cr level may remain in the normal range despite a major decline in GFR[4,23]. Moreover, several studies have shown that CLCr overestimates true GFR about 13 mL/min per 1.73 m2 compared to inulin clearance in patients with cirrhosis[24,25]. Variation in creatinine excretion exists during the day, making estimation of GFR, even from a valid 24-hour urine collection, incorrect[25].
Some studies have indicated that serum CysC could be proposed as a marker of liver disease stage and a more sensitive indicator of renal function in patients with cirrhosis than serum Cr level[26-28].
Particularly in patients with Child-Pugh class C, CysC determination is a valuable tool for the early diagnosis of moderately impaired renal function[5].
Significant differences were observed in CysC values but not Cr values, between Child-Pugh class A, B, and C. The finding suggests that CysC may indirectly reflect the degree of liver dysfunction[22,29]. Woitas et al[30] found that CysC was significantly higher in Child-Pugh B and C patients when compared to Child-Pugh A patients. Still no difference was observed between patients with Child-Pugh B and C. Similar to the previous findings, we confirmed that the values of CysC were significantly increased in advanced stages (Child-Pugh B and C) compared to early stage (Child-Pugh A) of liver cirrhosis, although there was no significant difference between B and C stages. Additionally, we report a significant correlation between CysC and MELD score, advocating CysC as a valid marker of liver insufficiency. Considering a variety of non-renal factors influencing serum creatinine levels, and exclusion of patients with HRS from the study, no significant correlation between Cr and MELD score was noted. Prognostic and clinical significance of CysC is also shown in a recent study stating that CysC, serum sodium and prothrombin time were independent factors for predicting survival in patients with cirrhosis[31].
In our study, unlike Cr, CysC levels and serum and urinary concentration of sodium demonstrated a strong negative correlation, suggesting a clinical relevance of CysC. These findings may be especially useful for monitoring patients with decompensated liver cirrhosis.
A study comparing GFRCys formula to GFRCr formula showed that CysC was more likely to predict the patients’ GFR below or above 60 mL/min per 1.73 m2[32]. Furthermore, the CysC showed a more significant correlation than serum Cr with GFR by 99mTc-DTPA technique[4,33,34]. Also, Coll et al[35] reported that serum CysC levels started to increase when GFR was 88 mL/min per 1.73 m2, while serum Cr level began to increase when GFR was 75 mL/min per 1.73 m2.
However, Xirouchakis et al[36] compared 51Cr-EDTA with GFRCr and GFRCys formulas in 74 patients with cirrhosis, candidates for liver transplantation. They reported that estimated GFR in cirrhosis is not better based on CysC formulas compared with creatinine ones.
In contrast, we identified significantly more patients with decreased glomerular filtration based on GFRCys compared to GFRCr. In regards to our findings, we suggest GFRCys as a more sensitive parameter for assessment of renal function in patients with liver cirrhosis.
The RRI is a sensitive marker of intrarenal hemodynamics and it has been reported to increase even in non-azotemic patients with cirrhosis[37]. Moreover, data suggest that normal serum Cr levels may be associated with a significantly decreased glomerular filtration rate and that more than 50% of patients with end-stage liver disease and increased RRI have normal serum creatinine levels[38,39].
A recently published study reported that RRI was significantly higher in Child-Pugh C patients than in Child-Pugh B or A patients[23]. Findings from our research showed that RRI significantly increased from Child-Pugh stages A to C. Also, the RRI increased with an increase of MELD score. These results indicate that RRI is directly influenced by liver insufficiency degree.
In cirrhotic patients fluid accumulation can occur (in form of pedal edema, minimal ascites and/or diuretic-sensitive ascites), and renal blood flow is expected to decrease with GFR maintained at normal levels by increased filtration fraction. The recognition and identification of these patients are particularly important for the early intervention and prevention of progression of renal diseases[40]. Accordingly, we aimed to examine this hemodynamic disturbance by measuring RRI.
To our knowledge, a small number of studies have been published on possible correlations between CysC, GFR and arterial renal blood flow resistance. Cystatin C was compared to RRI in patients with viral C cirrhosis and authors reported significant positive correlations[22]. Ustundag et al[29] noted that serum CysC, but not serum creatinine or RRI measurement, correlated with GFR (GFR was estimated by technetium (99m)-diethylene triamine pentaacetic acid renal scintigraphy), in each stage of liver failure. Interestingly, our data showed that RRI significantly correlated with GFRCys, but not with GFRCr.
In conclusion, CysC may be a more reliable marker for liver insufficiency assessment. Additionally, RRI and CysC represent sensitive indicators of renal dysfunction in patients with liver cirrhosis.
COMMENTS
Background
Early diagnosis of impaired renal function in cirrhotic patients is very important to prevent serious complications. Renal blood flow decrease is common, while glomerular filtration rate (GFR) remains normal because the filtration fraction increases. The protease inhibitor (CysC) has been proposed as a specific marker of GFR and an early indicator of impaired renal function.
Research frontiers
Some studies have indicated that serum CysC could be proposed as a marker of liver disease stage and a more sensitive indicator of renal function in patients with cirrhosis. Opposed to creatinine (Cr), CysC is independent of gender, age, and muscle mass and not influenced by serum bilirubin, inflammation, or malignancy. The renal resistive index (RRI) is proposed to be a sensitive marker of intrarenal hemodynamics in patients with cirrhosis. The research hotspot is to evaluate the clinical significance of cystatin C and RRI in early detection of renal dysfunction in cirrhotic patients.
Innovations and breakthroughs
Previous studies have shown different results. Some confirmed a significant correlation with CysC based GFR but not with Cr based GFR, when compared to technetium 99m-diethylene triamine pentaacetic acid renal scintigraphy technique. However, others compared GFRCr and GFRCys with chromium-51 labeled ethylenediamine tetraacetic acid in patients with cirrhosis and found no significant difference. These procedures that estimated GFR based on exogenous substances are invasive and carry a risk for patients, usually are considered too expensive and time consuming for routine clinical use. In order to eliminate these invasive and costly procedures, the authors measured RRI to evaluate renal blood flow, and examined correlations with GFRCys and GFRCr. RRI correlated significantly with CysC and showed a significant negative correlation with GFRCys. Moreover, the authors detected no relationship between RRI and Cr, CLCr, or GFRCr.
Applications
The study results suggest that RRI and CysC represent sensitive indicators of renal dysfunction in patients with liver cirrhosis.
Terminology
Cystatin C: CysC is a non-glycosylated 13 kDa protein, produced at a constant rate by all nucleated cells, freely filtered by the glomeruli and subsequently metabolized in the proximal tubules. Renal resistive index: RRI equals peak systolic velocity minus the final diastolic velocity divided by the peak systolic velocity. RRI less than 0.7 is considered normal and was calculated based on the mean value of renal arteries.
Peer review
The manuscript written by Culafic et al describes that cystatin C and renal resistive index may be more reliable markers for assessment of liver and renal dysfunction in patients with liver cirrhosis. Conventionally, renal dysfunction is assessed by serum Cr or GFRCr. However, cystatin C and renal resistive index are more sensitive than those markers. The manuscript provides important information in the management of patients with liver cirrhosis.
Footnotes
Supported by Ministry of Education, Science and Technological Development, Belgrade, Republic of Serbia, Project No. 175036
P- Reviewers: Privitera G, Shimizu Y, Stadlbauer V S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819–1827. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 2.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arroyo V, Fernandez J, Ginès P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008;28:81–95. doi: 10.1055/s-2008-1040323. [DOI] [PubMed] [Google Scholar]
- 4.Kim DJ, Kang HS, Choi HS, Cho HJ, Kim ES, Keum B, An H, Kim JH, Seo YS, Kim YS, et al. Serum cystatin C level is a useful marker for the evaluation of renal function in patients with cirrhotic ascites and normal serum creatinine levels. Korean J Hepatol. 2011;17:130–138. doi: 10.3350/kjhep.2011.17.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbes AL, Gülberg V, Bilzer M, Vogeser M. Evaluation of serum cystatin C concentration as a marker of renal function in patients with cirrhosis of the liver. Gut. 2002;50:106–110. doi: 10.1136/gut.50.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew JS, Saleem M, Florkowski CM, George PM. Cystatin C--a paradigm of evidence based laboratory medicine. Clin Biochem Rev. 2008;29:47–62. [PMC free article] [PubMed] [Google Scholar]
- 7.Newman DJ. Cystatin C. Ann Clin Biochem. 2002;39:89–104. doi: 10.1258/0004563021901847. [DOI] [PubMed] [Google Scholar]
- 8.Zahran A, El-Husseini A, Shoker A. Can cystatin C replace creatinine to estimate glomerular filtration rate? A literature review. Am J Nephrol. 2007;27:197–205. doi: 10.1159/000100907. [DOI] [PubMed] [Google Scholar]
- 9.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 10.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 11.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 12.Bistrian BR. Nutritional assessment and therapy of protein--calorie malnutrition in the hospital. J Am Diet Assoc. 1977;71:393–397. [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 14.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, Zhang YL, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satyapal KS, Rambiritch V, Pillai G. Morphometric analysis of the renal veins. Anat Rec. 1995;241:268–272. doi: 10.1002/ar.1092410213. [DOI] [PubMed] [Google Scholar]
- 16.Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol. 2003;180:885–892. doi: 10.2214/ajr.180.4.1800885. [DOI] [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 18.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 19.Herget-Rosenthal S, Bökenkamp A, Hofmann W. How to estimate GFR-serum creatinine, serum cystatin C or equations? Clin Biochem. 2007;40:153–161. doi: 10.1016/j.clinbiochem.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Peake M, Whiting M. Measurement of serum creatinine--current status and future goals. Clin Biochem Rev. 2006;27:173–184. [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41:269–278. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- 22.Pirlich M, Selberg O, Böker K, Schwarze M, Müller MJ. The creatinine approach to estimate skeletal muscle mass in patients with cirrhosis. Hepatology. 1996;24:1422–1427. doi: 10.1002/hep.510240620. [DOI] [PubMed] [Google Scholar]
- 23.El-Shazly M, Shayeb AE, Moez P, Sami M, Zaghloul M. Diagnostic Value of Serum Cystatin C as an Early Indicator of Renal Impairment in Chronic HCV Egyptian Patients with Liver Cirrhosis. J Am Sci. 2011;7:75–81. [Google Scholar]
- 24.Orlando R, Mussap M, Plebani M, Piccoli P, De Martin S, Floreani M, Padrini R, Palatini P. Diagnostic value of plasma cystatin C as a glomerular filtration marker in decompensated liver cirrhosis. Clin Chem. 2002;48:850–858. [PubMed] [Google Scholar]
- 25.Proulx NL, Akbari A, Garg AX, Rostom A, Jaffey J, Clark HD. Measured creatinine clearance from timed urine collections substantially overestimates glomerular filtration rate in patients with liver cirrhosis: a systematic review and individual patient meta-analysis. Nephrol Dial Transplant. 2005;20:1617–1622. doi: 10.1093/ndt/gfh839. [DOI] [PubMed] [Google Scholar]
- 26.Chung MY, Jun DW, Sung SA. Diagnostic value of cystatin C for predicting acute kidney injury in patients with liver cirrhosis. Korean J Hepatol. 2010;16:301–307. doi: 10.3350/kjhep.2010.16.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demirtaş S, Bozbaş A, Akbay A, Yavuz Y, Karaca L. Diagnostic value of serum cystatin C for evaluation of hepatorenal syndrome. Clin Chim Acta. 2001;311:81–89. doi: 10.1016/s0009-8981(01)00546-0. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi M, Fukuda Y, Nakano I, Katano Y, Hayakawa T. Elevation of serum cystatin C concentrations in patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2001;13:951–955. doi: 10.1097/00042737-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Ustundag Y, Samsar U, Acikgoz S, Cabuk M, Kiran S, Kulah E, Aydemir S. Analysis of glomerular filtration rate, serum cystatin C levels, and renal resistive index values in cirrhosis patients. Clin Chem Lab Med. 2007;45:890–894. doi: 10.1515/CCLM.2007.130. [DOI] [PubMed] [Google Scholar]
- 30.Woitas RP, Stoffel-Wagner B, Flommersfeld S, Poege U, Schiedermaier P, Klehr HU, Spengler U, Bidlingmaier F, Sauerbruch T. Correlation of serum concentrations of cystatin C and creatinine to inulin clearance in liver cirrhosis. Clin Chem. 2000;46:712–715. [PubMed] [Google Scholar]
- 31.Ahn HS, Kim YS, Kim SG, Kim HK, Min SK, Jeong SW, Jang JY, Lee SH, Kim HS, Kim BS, et al. Cystatin C is a good predictor of hepatorenal syndrome and survival in patients with cirrhosis who have normal serum creatinine levels. Hepatogastroenterology. 2012;59:1168–1173. doi: 10.5754/hge11616. [DOI] [PubMed] [Google Scholar]
- 32.Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L. Serum cystatin C-based equation compared to serum creatinine-based equations for estimation of glomerular filtration rate in patients with chronic kidney disease. Clin Nephrol. 2008;70:10–17. doi: 10.5414/cnp70010. [DOI] [PubMed] [Google Scholar]
- 33.El-Agroudy AE, Sabry AA, Ghanem HA, El-Baz A, Fakhry A, Gad HM, Sheashaa HA, Abdel-Hamid M, Yousseff M, El-Rahman A, et al. Serum cystatin C: A good marker for evaluation of glomerular filtration rate in hepatorenal syndrome. European J General Med. 2004;1:29–35. [Google Scholar]
- 34.Biancofiore G, Pucci L, Cerutti E, Penno G, Pardini E, Esposito M, Bindi L, Pelati E, Romanelli A, Triscornia S, et al. Cystatin C as a marker of renal function immediately after liver transplantation. Liver Transpl. 2006;12:285–291. doi: 10.1002/lt.20657. [DOI] [PubMed] [Google Scholar]
- 35.Coll E, Botey A, Alvarez L, Poch E, Quintó L, Saurina A, Vera M, Piera C, Darnell A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 36.Xirouchakis E, Marelli L, Cholongitas E, Manousou P, Calvaruso V, Pleguezuelo M, Guerrini GP, Maimone S, Kerry A, Hajjawi M, et al. Comparison of cystatin C and creatinine-based glomerular filtration rate formulas with 51Cr-EDTA clearance in patients with cirrhosis. Clin J Am Soc Nephrol. 2011;6:84–92. doi: 10.2215/CJN.03400410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maroto A, Ginès A, Saló J, Clària J, Ginès P, Anibarro L, Jiménez W, Arroyo V, Rodés J. Diagnosis of functional kidney failure of cirrhosis with Doppler sonography: prognostic value of resistive index. Hepatology. 1994;20:839–844. doi: 10.1002/hep.1840200411. [DOI] [PubMed] [Google Scholar]
- 38.Gentilini P, Laffi G. Renal functional impairment and sodium retention in liver cirrhosis. Digestion. 1989;43:1–32. doi: 10.1159/000199857. [DOI] [PubMed] [Google Scholar]
- 39.Platt JF, Marn CS, Baliga PK, Ellis JH, Rubin JM, Merion RM. Renal dysfunction in hepatic disease: early identification with renal duplex Doppler US in patients who undergo liver transplantation. Radiology. 1992;183:801–806. doi: 10.1148/radiology.183.3.1584937. [DOI] [PubMed] [Google Scholar]
- 40.Mindikoglu AL, Weir MR. Current concepts in the diagnosis and classification of renal dysfunction in cirrhosis. Am J Nephrol. 2013;38:345–354. doi: 10.1159/000355540. [DOI] [PMC free article] [PubMed] [Google Scholar]


