FIGURE 6.
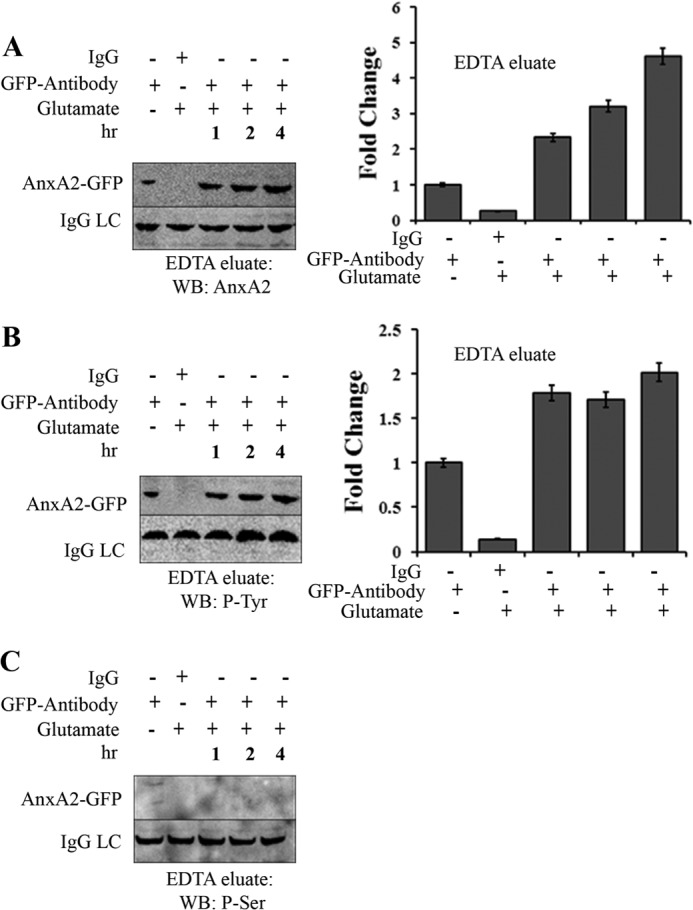
Glutamate-induced cell surface AnxA2 is tyrosine-phosphorylated. A, 661W cells transfected with a plasmid vector expressing wild-type AnxA2-GFP were treated with 500 μm glutamate for 1, 2, and 4 h. The cells were incubated in EDTA (Invitrogen) for 20 min. The EDTA eluates were immunoprecipitated with anti-GFP antibody, subjected to SDS-PAGE, and analyzed by Western blotting (WB) with anti-AnxA2 antibody. The eluates were incubated with nonspecific anti-mouse IgG as negative controls. LC, light chain. B and C, immunoprecipitates of the EDTA eluates collected as mentioned above were immunoblotted with anti-phosphotyrosine and anti-phosphoserine antibodies. The IgG light chain of the antibody used for immunoprecipitation was used as a control for loading. All blots were exposed to identical exposure times. The fold change to the respective GFP antibody treatments is shown. C, because we could not detect any serine-phosphorylated (P-Ser) AnxA2-GFP in the EDTA eluates of glutamate-treated and untreated cells, it could not be quantified. P-Tyr, tyrosine-phosphorylated.
