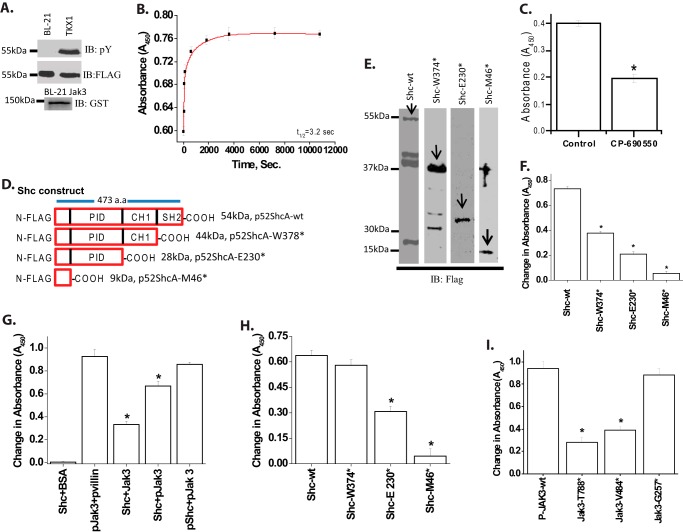
FIGURE 1.
Recombinant Jak3 trans-phosphorylates adapter proteins p52ShcA. A, the expression and tyrosine phosphorylation of recombinant proteins were detected through Western analysis using FLAG, phosphotyrosine (pY), and GST antibody. B, changes in tyrosine trans-phosphorylation of ShcA-wt by Jak3-wt were detected in the presence or absence (control) of ATP using 96-well microtiter plates precoated with FLAG-ShcA-wt proteins, and the phosphorylation was induced by the addition of activated Jak3 (P-Jak3-wt) (6), where P-Jak3-wt alone and FLAG-ShcA-wt alone were taken as controls. The phosphorylation was detected as reported before (6). Curve-fitting was done as reported before (25) using the Hyperbol-fit program in MicroCal Origin to calculate t½. C, similar experiments were performed as in B except in the presence of Jak3 inhibitor CP-690505 and a fixed reaction time of 5 min. D, schematic representation of FLAG-p52ShcA-wt and mutants. E, wt and mutants were expressed and purified as in A, and Western analysis of the expressed proteins was done using anti-GST antibody. Arrows indicate recombinant protein expression. F, similar experiments were performed as in C using different truncation mutants except that the 96-well microtiter plates were precoated with p52ShcA-wt or its truncation mutants and the phosphorylation was induced by the addition of P-Jak3-wt where P-Jak3-wt alone and Shc-wt or mutants alone were taken as controls. G, direct interactions between Jak3-wt and Shc-wt were determined by a pairwise binding assay (detailed in supplemental Fig. S3) except using equimolar concentrations of the indicated proteins with BSA as negative and villin as positive control. H, direct interactions between Jak3-wt and Shc mutants were determined as in G. I, direct interactions between P-Shc-wt and the indicated Jak3 mutants were determined as in H. A and E, blots are representative from n = 3 experiments. B and C and F–I, values are mean ± S.E.* indicates statistically significant differences from control (in C), Shc-wt (in F and H), P-Shc-P-Jak3 (in G), and P-Jak3 (in I); p < 0.05, n = 3 experiments.