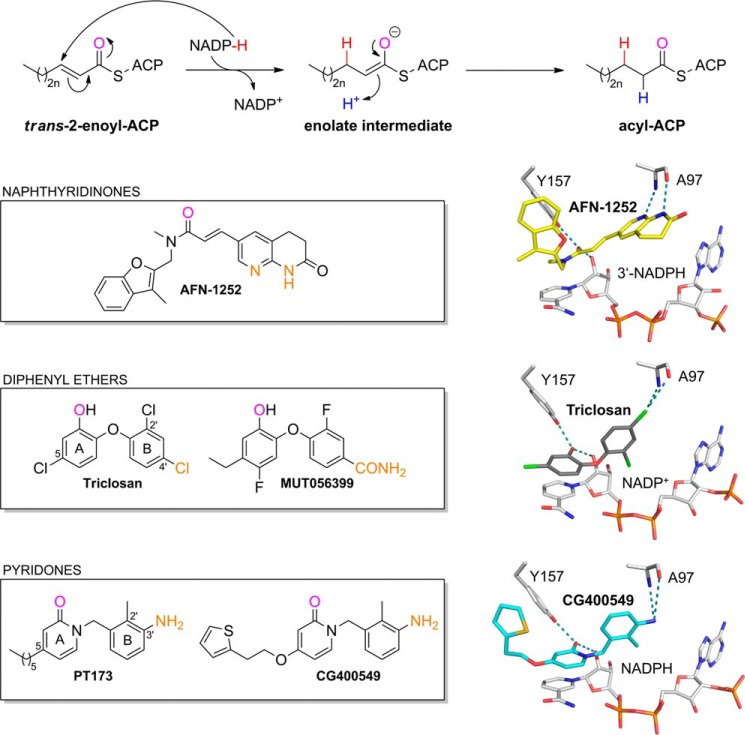
FIGURE 1.
Catalyzed reaction and successful inhibitor classes of S. aureus FabI. FabI catalyzes the reduction of the trans-2-enoyl-ACP substrate via an enolate intermediate (n = 0–8) (78). In the case of saFabI, the hydride (shown in red) is delivered by the reducing agent NADPH (6, 8, 9). During the second step of the reaction, the enolate intermediate is protonated, which leads to the formation of the final acyl-ACP product (the proton is depicted in blue). Examples for the most important FabI inhibitor classes are given in the boxes along with their binding mode in the saFabI active site pocket (PDB codes 4FS3 and 4ALI (6, 23); the CG400549 structure was solved during this study, PDB code 4CV1). For each of these inhibitor scaffolds, one compound is currently in clinical trials (AFN-1252, MUT056399, and CG400549) (25). One common feature of these FabI inhibitors is the formation of a hydrogen bond to Tyr-157 and the cofactor NADP(H). The oxygen atoms involved in this central interaction are colored in magenta. In addition, all depicted inhibitors directly interact with Ala-97 (the interacting inhibitor atoms are highlighted in orange).