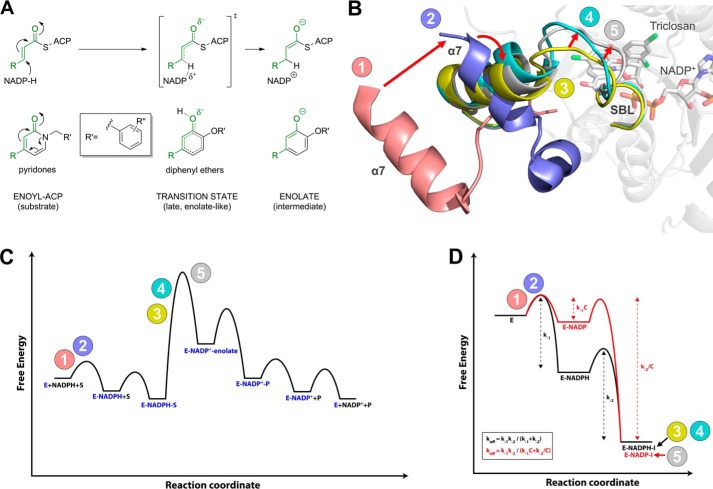
FIGURE 9.
Conformational states sampled along the reaction coordinates of inhibitor binding and substrate turnover. A, pyridone and diphenyl ether inhibitors resemble different species along the enzymatic reaction coordinate. In contrast to the enolate-like diphenyl ethers which are transition state analogues (31), pyridones are more substrate-like. The corresponding moieties of inhibitors and species along the reaction coordinate are highlighted in green. B, conformational states sampled by the SBL and the attached helix α7 of saFabI. Red arrows indicate the conformational changes we propose to occur during the enzymatic reaction (see also C). Prior to the binding of cofactor and substrate or inhibitor, the SBL is disordered, and helix α7 attains a very open conformation (state 1 colored in pink = PDB code 4ALM, subunit B; state 2 colored in lilac = PDB code 4ALM, subunit C; further details about the conformational changes upon ligand binding are provided in our previous report (6)). The more substrate-like pyridone inhibitors likely induce a conformational state between the ternary E-NADPH-S complex and the transition state of the hydride transfer (state 3 colored in yellow = CG400549-I structure, subunit C; state 4 colored in cyan = CG400549-I structure, subunit A; see also Fig. 5B). In contrast, the transition state analogue triclosan and several other diphenyl ethers (6, 31) induce the likely fully closed state of the SBL (state 5 colored in gray = PDB code 4ALI, subunit H). C, qualitative energy diagram for substrate turnover by saFabI. The numbers 1–5 indicate the conformational states (colors according to B), which are likely sampled along the reaction coordinate of the enzymatic reaction (see also B). D, approximate energy diagrams for saFabI in complex with NADPH and pyridone (black) or NADP+ and diphenyl ether inhibitor (red). The overall affinities of both ternary complexes are assumed to be identical. By shifting stabilization from cofactor to inhibitor, the residence time of the overall complex is increased. This rationalizes the difference in off-rate kinetics between the diphenyl ethers and pyridones. Note that, technically, koff for the pyridone complex (E-NADPH-I) is equal to k−2 because the E-NADPH complex is catalytically active. C is defined as a constant with a value greater than 1. The numbers 1–5 indicate the conformational states (colors according to B), which are likely sampled along the reaction coordinate of inhibitor binding (see also B).