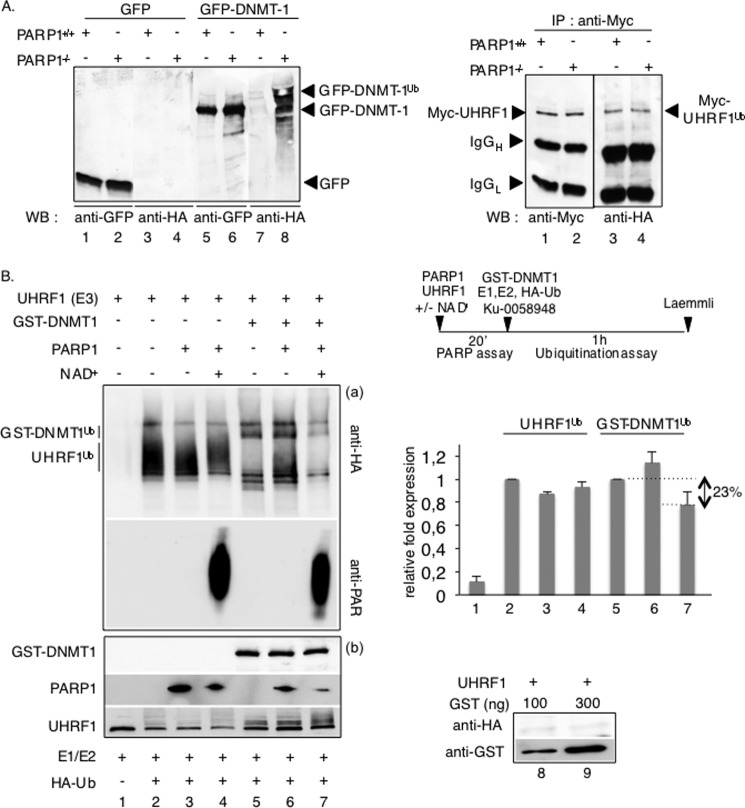
FIGURE 7.
PARP1 selectively inhibits the UHRF1-driven ubiquitination of DNMT1 in vivo and in vitro. A, in vivo ubiquitination assays. The absence of PARP1 enhances the UHRF1-mediated ubiquitination of DNMT1 but not the autoubiquitination of UHRF1 in vivo. Left, PARP1+/+ and PARP1−/− cells were transfected with either GFP-DNMT1 (lanes 5–8) or GFP (lanes 1–4) together with HA-ubiquitin and treated with 5 μm MG-132 for 12 h to inhibit proteasomal degradation. GFP immunoprecipitates were blotted successively with an anti-HA antibody to detect ubiquitinated proteins (lanes 3, 4, 7, and 8) and an anti-GFP antibody (lanes 1, 2, 5, and 6) to detect immunopurified proteins. Right, PARP1+/+ (lanes 1 and 3) and PARP1−/− cells (lanes 2 and 4) were transfected with Myc-UHRF1 together with HA-ubiquitin and treated as described above. Myc immunoprecipitates were blotted successively with an anti-HA antibody to detect ubiquitinated UHRF1 (lanes 3 and 4) and an anti-Myc antibody to detect immunopurified UHRF1 (lanes 1 and 2). B, in vitro ubiquitination assays. Left, PARP1-catalyzed poly(ADP-ribosyl)ation of UHRF1 selectively inhibits its ubiquitination activity onto DNMT1. Purified UHRF1 was first preincubated alone (lanes 1, 2, and 5) or together with purified PARP1 (lanes 3, 4, 6, and 7) as indicated in the PARP activity buffer. PARP activity is induced by the addition of NAD+. The proteins were subsequently assayed for UHRF1 ubiquitination activity onto itself (lanes 1–4) or onto GST-DNMT1 (lanes 5–7). a, ubiquitinated proteins (UHRF1Ub and GST-DNMT1Ub) were detected by immunoblotting using an anti-HA antibody, and the PARP activity was verified by immunoblotting using an anti-PAR antibody. b, the purified recombinant proteins mixed in the experiment were detected by Western blotting using anti-GST, anti-PARP1, and anti-UHRF1 antibodies. The lower amount of PARP1 detected in lanes 4 and 7 is explained by its automodification, which limits its detection by the monoclonal anti-PARP1 antibody used. As a control, reactions were performed with GST (lanes 8 and 9). A representative experiment of three is shown. Upper right, a schematic diagram of the experiment is shown. Lower right, the relative -fold expression (histogram) represents the ImageJ-quantified ubiquitinated protein levels of the samples containing PARP1 relative to the samples without PARP1 (lanes 1, 3, and 4 versus 2; lanes 6 and 7 versus 5). The values represent the mean ± S.D. of three independent experiments.