FIGURE 4.
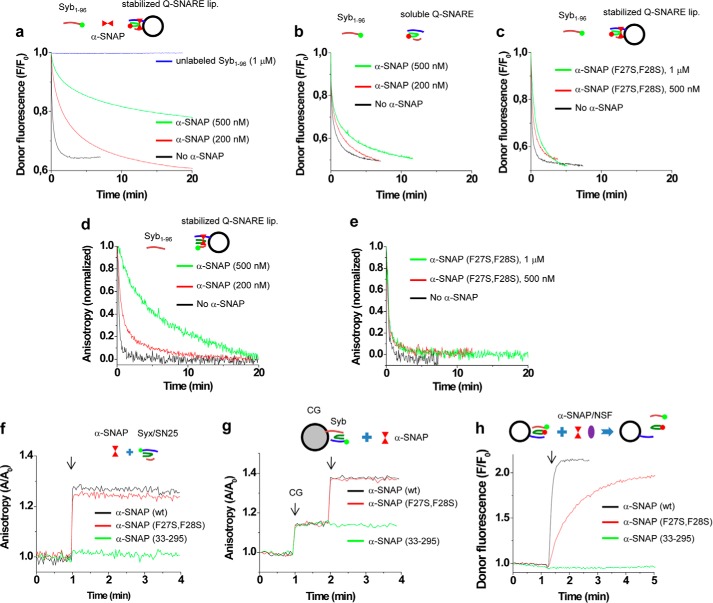
α-SNAP interferes with SNARE assembly. a, SNARE assembly was monitored using FRET in which soluble Syb(1–96) and SNAP-25A of the stabilized Q-SNARE complex were labeled with Oregon Green and Texas Red, respectively (see “Experimental Procedures” for details). The reaction was initiated by adding 40 nm labeled Syb(1–96) to liposomes containing labeled SNAP-25A. α-SNAP retarded SNARE assembly kinetics in a dose-dependent manner. b and c, membrane attachment of α-SNAP is critical for retardation of SNARE assembly. SNARE complex was not incorporated in liposomes (b). d and e, as an alternative assay, SNARE assembly was also monitored by following dissociation of the stabilizing synaptobrevin-2 peptide (Syb(49–96)) using fluorescence anisotropy. Syb(49–96) in the stabilized Q-SNARE complex was labeled with Alexa Fluor 488 (Syb(49–96)A488) and incorporated in liposomes. The liposomes were preincubated for 5 min with WT α-SNAP (d) or α-SNAP (F27S, F28S) (e), followed by the addition of 50 nm unlabeled Syb(1–96) to monitor peptide dissociation as a read-out for SNARE complex formation. f-h, the mutants α-SNAP (F27S, F28S) and α-SNAP(33–295) differ in their ability to bind and disassemble membrane-anchored SNARE complexes. Binding of the α-SNAP variants was monitored by fluorescence anisotropy using either the soluble stabilized Q-SNARE complexes (consisting syntaxin-1A(183–262), SNAP-25A, and Syb(49–96)) (f) or membrane-anchored ternary complexes on CGs (endogenous synaptobrevin-2 displaces Syb(49–96)) (g), where syntaxin-1A was labeled at position Cys225 with Alexa Fluor 488. Note that under both conditions binding of WT α-SNAP and α-SNAP (F27S, F28S) was observed, whereas α-SNAP(33–295) did not bind. h, disassembly was monitored by FRET using membrane-anchored complexes as in a, in which Syb(1–96) and SNAP-25A of the stabilized Q-SNARE complex were labeled with Oregon Green and Texas Red, respectively. Dissociation was monitored by an increase in donor fluorescence due to the dissociation of SNAP-25A from the complex. 100 nm NSF and 5 mm ATP were included in buffer and 5 mm MgCl2 was added to activate disassembly (arrow). The concentration of the α-SNAP variants (f–h) was 500 nm.