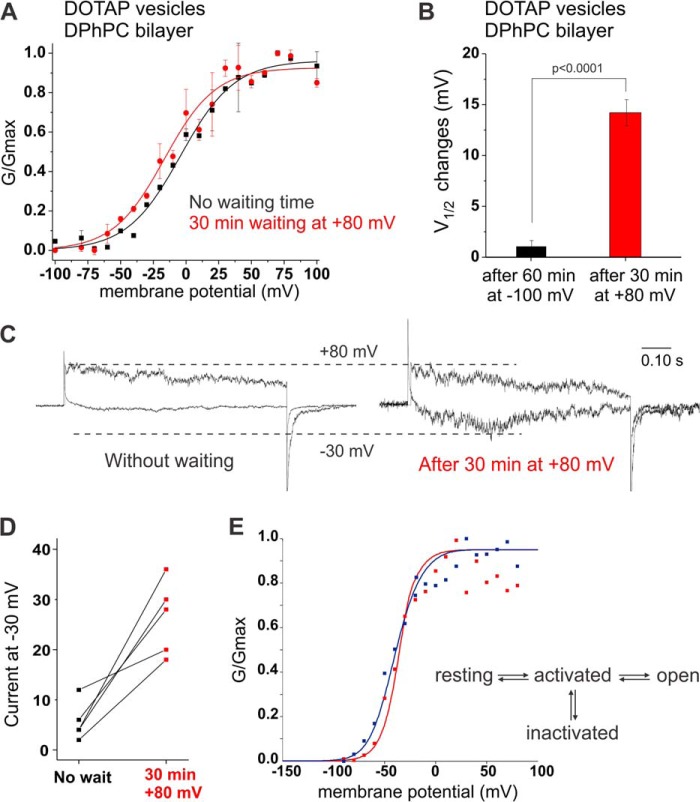
FIGURE 4.
State-dependent accessibility of lipid binding site. A, KvAP reconstituted in DOTAP vesicles were fused to a DPhPC bilayer. Voltage pulses ranging from −100 to +100 mV were applied immediately after fusion (black) or after waiting 30 min at +80 mV (red). Holding at this depolarizing pulse promoted a shift of the midpoint of activation by −12.6 mV. V½ and VS values are presented in Table 2, n = 5, error bars are S.D. B, changes observed in the V½ values when KvAP channels reconstituted in DOTAP vesicles and fused to a DPhPC bilayer are held 60 min at −100 mV (black) and 30 min at +80 mV (red, p < 0.0001). C, example of current traces at −30 mV compared with +60 mV before and after 30 min waiting time at +80 mV. D, normalized currents at −30 mV for each bilayer experiment show an increase in the open probability after waiting 30 min at +80 mV. E, kinetic model for the gating behavior of KvAP (27). The transition from the resting to the activated state is the major charge carrying step. In the graph, the GV for KvAP reconstituted in DPhPC vesicles and fused to DPhPC (red) and DOTAP:DPhPC, 2:1 (blue), are shown. The data has been fitted to the kinetic model, where the blue fit has a 2.3-fold lower energy barrier for transitions from the activated to the open and inactivated state.