FIGURE 5.
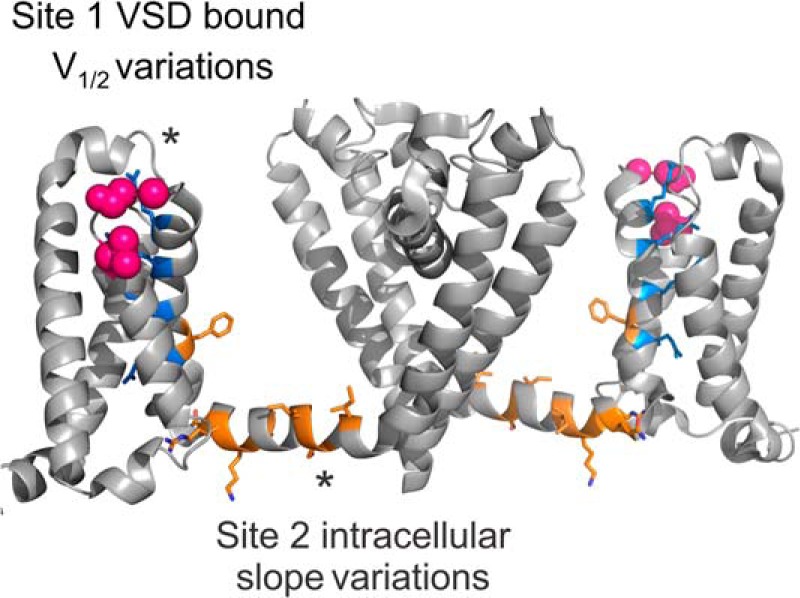
Model for lipid binding sites in KvAP. Side view of 2 subunits of the sodium channel NavAb (PDB 4EKW) (40) are shown, with gating arginines colored in blue. We located the site responsible for the V½ variations at the extracellular region of the voltage sensor, possibly at the S3–S4 loop, near the first gating charge. This region (pink spheres) has already been shown to strongly interact with lipids, and has also been identified for sphingomyelin based on competitive interaction with spider toxins (8–10, 14, 16, 17, 25, 26). We located the second site at the intracellular side of the channel. The residues in orange correspond to those shown to interact with lipids in the crystal structure of the Kv1.2/2.1 chimera (2). Lipids interacting in this region could affect the energy barriers of pore opening and entry into the inactivated state, possibly by interacting with the S4–S5 linker.
