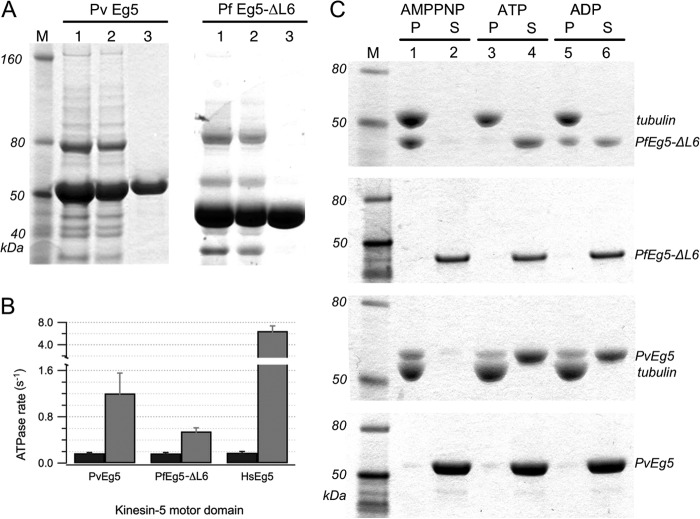
FIGURE 2.
Purification and characterization of PvEg5 and PfEg5-ΔL6. A, left, fractions from PvEg5 purification; right, fractions from PfEg5-ΔL6 purifications. Lanes 1 and 2 of both panels show elution fractions from nickel column purification and desalting steps, respectively. Lane 3 in both panels shows S-column elution fraction for each motor, with over 90% purity. B, basal (black) and MT-stimulated (gray) ATPase activity for motor domains of PvEg5, PfEg5-ΔL6, and HsEg5. C, Coomassie-stained SDS-PAGE of microtubule co-sedimentation assays and molecular weight markers (M). Microtubules were incubated with Plasmodium motor domains treated with AMPPNP, ATP, and ADP, respectively. Insoluble pellet (P) fractions were separated from the soluble supernatant (S) fractions. The top gel shows PfEg5-ΔL6 partitioning into the microtubule pellet fractions or soluble fractions depending on nucleotide treatment as marked. The second gel shows the partitioning behavior of the treated PfEg5-ΔL6 motor domain without any added microtubules. The third and fourth gels similarly show treated PvEg5 motor domain with and without added microtubules, respectively. Error bars, S.D.