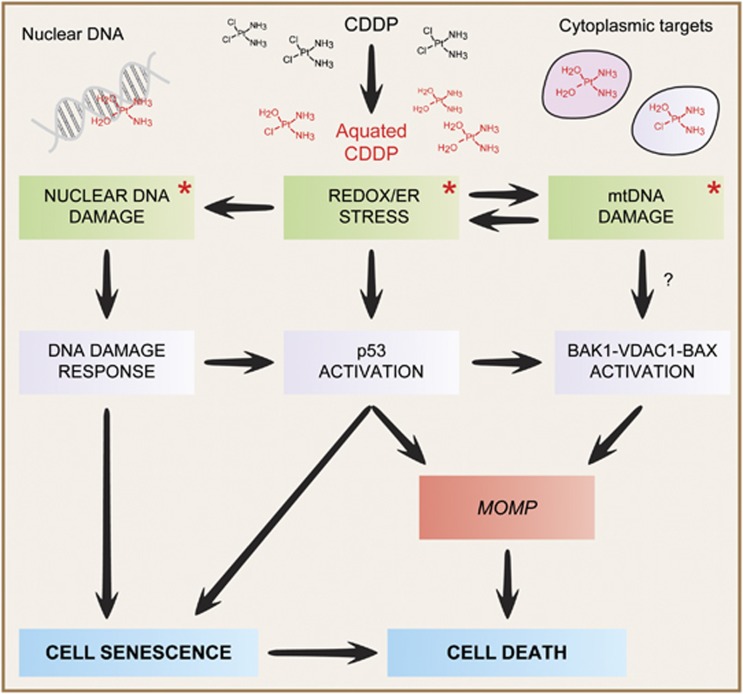
Figure 1.
Mode of action of cisplatin. As a result of the reduced cytoplasmic concentration of chloride ions, intracellular cisplatin (CDDP) is rapidly ‘aquated', hence acquiring a pronounced electrophilic reactivity. Aquated CDDP binds with high affinity to nuclear DNA, in particular to nucleophilic N7 sites on purines, thereby promoting the activation of the DNA damage response. In addition, CDDP can physically interact with several cytoplasmic nucleophiles, including mitochondrial DNA (mtDNA) as well as multiple mitochondrial and extramitochondrial proteins, hence (i) favoring the establishment of oxidative and reticular stress; (ii) eliciting a signal transduction cascade that involves the pro-apoptotic BCL-2 family members BAK1 and BAX, as well as voltage-dependent anion channel 1 (VDAC1) and (iii) activating the cytoplasmic pool of p53. The relative contribution of these nuclear and cytoplasmic modules to the cytostatic/cytotoxic activity of CDDP remains to be precisely elucidated and may exhibit an elevated degree of context dependency. Asterisks tag the primary consequences of CDDP reactivity. ER, endoplasmic reticulum; MOMP, mitochondrial outer membrane permeabilization