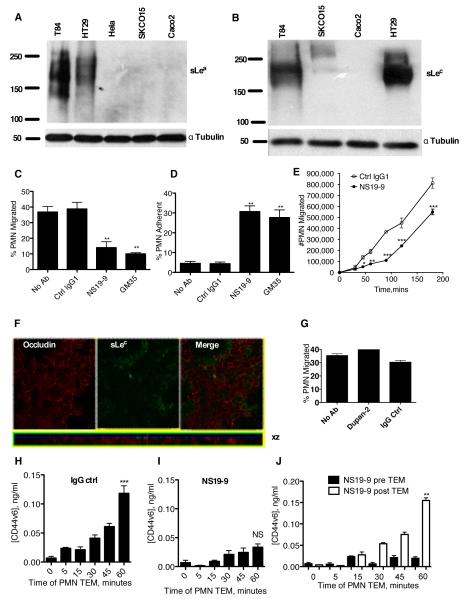
Figure 2. mAb NS19-9 but not Dupan-2 blocks PMN TEM and CD44v6 shedding.
Indicated cells were lysed and proteins immunoblotted with NS19-9 (A) or Dupan-2 (B). Confluent T84 monolayers were pre-treated with 10μg/ml NS19-9, 10μg/ml GM35, or 10μg/ml non-inhibitory IgG1 isotype control mAb before 1×106 PMNs were added to the basolateral surface. PMNs were allowed to migrate for 1 hour in response to a 100nM gradient of fMLF. The number of migrated PMNs (C) and the number of PMNs which were adherent to the apical epithelial surface (D) were quantified by myeloperoxidase assay. Data depict means ± SE (N=3). (E) Confluent T84 monolayers were treated apically with (closed circle) or without (open circle) 10μg/ml NS 19-9 before the addition of 1×106 PMN. PMN transmigration was then measured at the indicated time points. Data are mean +/− SE (n=3). (F) Confluent T84 monolayers were co-stained with 10μg/ml anti-Occludin mAb and 10μg/ml Dupan-2 and analyzed by confocal microscopy. (G) Confluent T84 monolayers were pre-treated with 10μg/ml Dupan-2 or 10μg/ml non-inhibitory IgG isotype control mAb before 1×106 PMNs were added to the basolateral surface. PMNs were allowed to migrate for 1 hour in response to a 100nM gradient of fMLF. The numbers of migrated PMN were quantified by myeloperoxidase assay. Data are means ± SE (N=3). 1×106 PMNs were added to confluent T84 monolayers treated apically with 10μg/ml binding control IgG1 (H) or 10μg/ml NS19-9 (I). PMNs were allowed to migrate in the basolateral to apical direction in response to a 100nM gradient of fMLF. At the time-points indicated the T84 containing filters were removed, and the solution from the apical migration reservoir assayed for soluble CD44v6 by ELISA. (J) Addition of mAb NS19-9 to the apical migration reservoir following PMN TEM does not prevent binding of released CD44v6 to the capture or detection ELISA mAbs. Significance was defined as p<0.05 (*, p<0.05; **, p<0.01; ***, p<0.001).