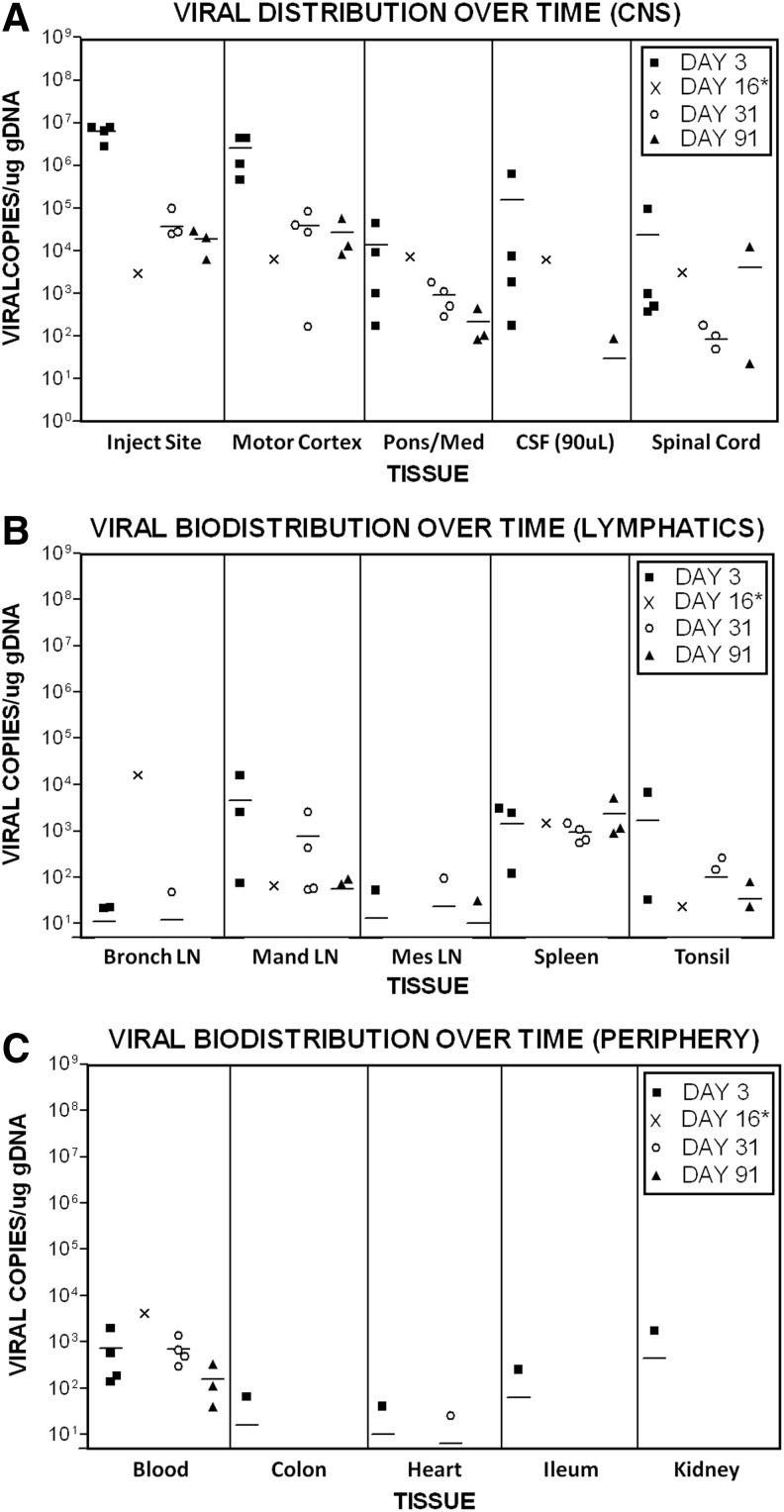
FIG. 5.
Viral biodistribution over time. The biodistribution of M032 after intracerebral administration was evaluated in NHP groups 1 (vehicle) and 3 (1×108 pfu M032). Plots are organized into (A) central nervous system tissues, including the injection site, motor cortex, pons/medulla oblongata (med), cerebral spinal fluid (CSF), and spinal cord; (B) lymphatic tissues (Bronch LN, bronchial lymph node; Man LN, mandibular lymph node; Mes LN, mesenteric lymph node); and (C) peripheral tissues. PCRs were monitored for contamination and the ability to amplify DNA through the use of both negative and positive control primer and probe sets within each reaction. The quality of samples was monitored by spiking one replicate of each sample with 50 copies of M032 vDNA and through the use of an internal amplification control. Data points represent the mean copy number of two replicates in each tissue per μg of gDNA (or per 90 μl for CSF). Less than 5 copies per μg gDNA represented the limit of detection. Horizontal bars represent the mean. Values obtained from the tissues of male NHP #907 euthanized moribund on day 16* are represented as “X.” PCRs, polymerase chain reactions.