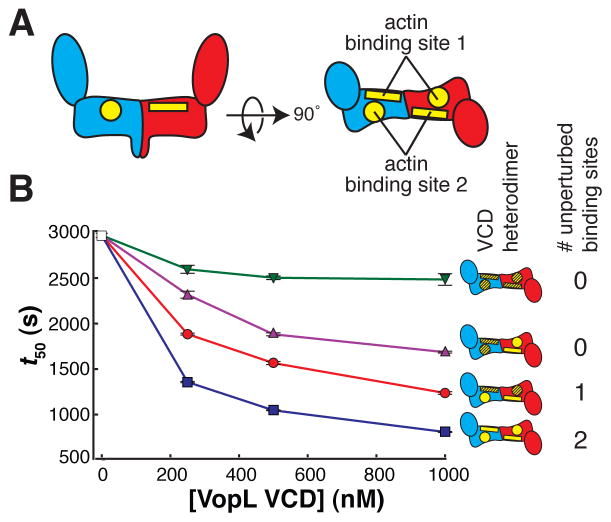
Figure 6. The VopL VCD can bind to the actin trimer in two equivalent orientations. See also Figure S6.
(A) Schematic side (left) and top (right) views of the VCD. The VCD homodimer has two symmetry-related binding sites for actin 1. We subdivide each of these binding sites into two surfaces, one residing on each VCD chain. One surface is shown as a rectangle (including residues E251, Y275, V405, and E408), and the other surface is shown as a circle (including residues N397, Y425, and R428).
(B) Actin assembly was quantified by measuring the time to half-maximal polymerization (t50). The average and 1σ standard error (n = 4, error bars appear in front of the symbol) are plotted for four heterodimers possessing different numbers of unperturbed actin binding sites (wild type sites and mutant sites are yellow and hatched, respectively). Both patches on both monomers represented by green triangles, both patches on one monomer by magenta triangles, one patch on one monomer and one patch on the other monomer by red circles, and the unmutated heterodimer by blue squares. Experiments using 0 nM VopL (white square) were included in both data sets, but are the same data.