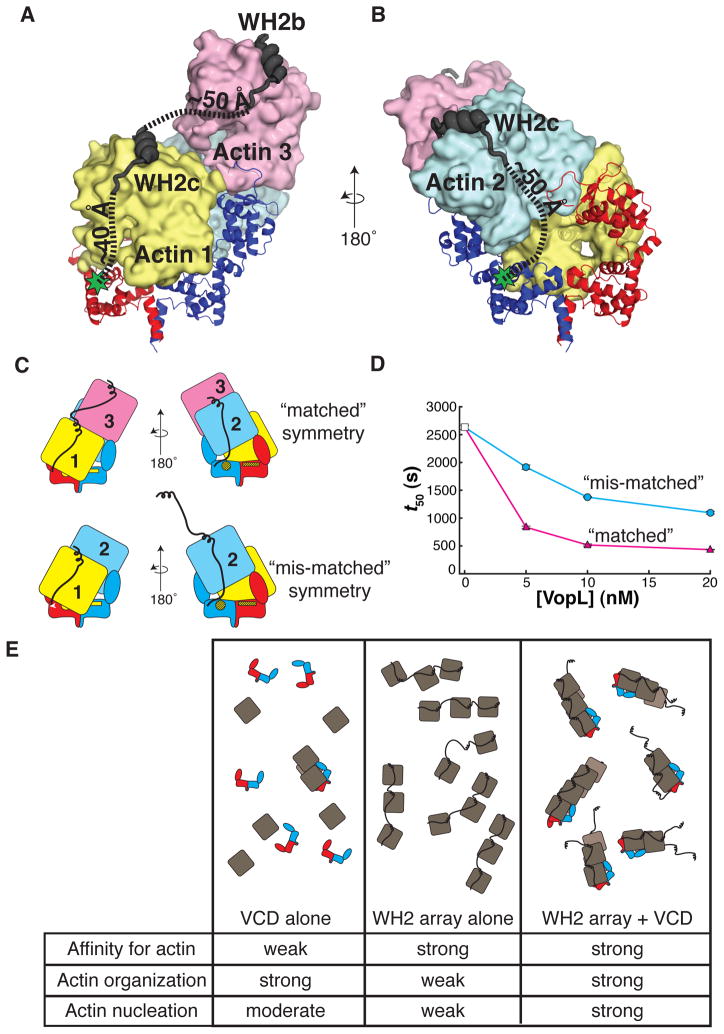
Figure 7. The VopL WH2 domains recruit and deliver actin monomers to the VCD.
(A) WH2b and WH2c modeled onto actin 3 and actin 1, respectively.
(B) WH2c modeled onto actin 2. (A and B) Green stars indicate the position of the VCD N-terminus. Dashed lines approximate the trajectories of the paths used to estimate the distances between structured elements in the model (VCD N-terminus to WH2c, and WH2c to WH2b).
(C) Illustration of VopL heterodimers that harbor mutations in the VCD that disrupt binding of actin 1 to one face, and additionally, are fused to a total of three WH2 motifs, an array of two WH2 motifs on one VCD monomer and a single WH2 motif on the other. The respective VCD monomers within a heterodimer are red or blue. Actins 1 (yellow), 2 (blue) and 3 (pink) are indicated. The unmutated face of the VCD is denoted by bright yellow patches. The mutated face of the VCD appears as yellow patches with black hash marks. Only the unmutated face could bind actin 1 in the mode observed in the crystal structure. The WH2 arrays are represented as black lines that emanate from the respective VCD monomers. In the construct with “matched” symmetry, the (WH2)2 array delivers actins 1 and 3 such that actin 1 contacts the unmutated face of the VCD; the single WH2 motif delivers actin 2, creating a stable trimer (Sept and McCammon, 2001). In the construct with “mis-matched” symmetry, if the system uses the wild type face of the VCD, there is no WH2 motif to deliver actin 3 longitudinally to actin 1; the (WH2)2 array could deliver an actin longitudinally to actin 2 (not shown in cartoon), but this would not create a stable actin trimer.
(D) Actin assembly was quantified by measuring the time to half-maximal polymerization (t50). The average and 1σ standard error (n = 4, error bars appear in front of the symbol) are plotted for two different VopL heterodimers. Both heterodimers harbor mutations that disrupt a single face of the VCD, and each possessing a single WH2 motif fused to one VCD monomer and two tandem WH2 motifs on the other. However, the two heterodimers differ from one another in how the WH2 arrays are positioned relative to the mutated surface on VCD, referred to as ‘matched’ (magenta triangles) or ‘mis-matched’ (cyan circles) as shown in panel C. Experiments using 0 nM VopL (white square) were included in both data sets, but are the same data.
(E) A summary of the roles of the VCD and the WH2 arrays in VopL-mediated actin nucleation. The cartoons represent the ensemble behavior, in the presence of actin, for the VCD alone, the WH2 array alone, and full length VopL. The rows in the table indicate the capacity to bind to actin, to organize actin into filament-like structures, and to nucleate actin filaments. Note that for visual clarity, in panels C and E, we represent the unbound WH2 motif as helical, although physical data suggest that in the free state WH2 motifs are disordered.