Abstract
Rats emit aversive taste reactivity (TR) behavior (i.e., gapes) following intraoral delivery of a cocaine-paired taste cue and greater conditioned aversive TR at the end of training predicts greater drug-seeking and taking. Here, we examined the development of this conditioned aversive TR behavior on a trial by trial basis in an effort to determine when the change in behavior occurs and whether early changes in this behavior can be used to predict later drug-taking. The results show that conditioned aversive TR to a cocaine-paired cue occurs very early in training (i.e., following as few as 1 – 2 taste-drug pairings) and, importantly, that it can be used to predict later drug-seeking and drug-taking in rats.
Keywords: cocaine, self-administration, taste reactivity, aversion, reward comparison
According to figures provided by the National Institute on Drug Abuse, in 2011, 68.2 million Americans had used tobacco, 18.1 million had used marijuana, 1.4 million had used cocaine, 1 million had used hallucinogens, and 0.3 million had used heroin, at least once in the month before the survey (Substance Abuse and Mental Health Services Administration, 2012). These numbers, while large, underestimate the scope of the problem because drug use often leads to addiction and addiction is a brain disease of chronic relapse (Leshner & Koob, 1999) costing society an estimated $484 billion per year (National Institute on Drug Abuse). Along with society, the addict and his or her family also suffer because substance abuse, dependence, and addiction are associated with an apparent devaluation of, and inattention to, natural rewards. Indeed, according to the DSM-IV, substance abuse and dependence involve a failure to fulfill major obligations at work, school, or home, the giving up of important social, occupational, or recreational activities, and continued drug use in spite of recurrent physical, legal, social, or psychological problems (American Psychiatric Association, 2000; Goldstein et al., 2007; Jones, Casswell, & Zhang, 1995; Nair et al., 1997; Santolaria-Fernandez et al., 1995).
This devastating consequence of drug addiction has been our focus for several years. Thus, we have honed an animal model for the systematic study of drug-induced devaluation of natural rewards. In our model, brief access to an otherwise palatable sweet, usually saccharin, is followed by access to either saline or a drug of abuse such as morphine, cocaine, or heroin (either experimenter- or self-administered). Following repeated daily pairings, rats come to avoid intake of the saccharin cue in anticipation of drug availability relative to intake by saccharin-saline paired controls. This paradigm has, in fact, been studied for decades (Cappell & LeBlanc, 1971; Le Magnen, 1969), but was quickly interpreted as a conditioned taste aversion akin to that induced by the illness-inducing agent, LiCl (Davis & Riley, 2010; Lester, Nachman, & Le Magnen, 1970), and, as such, as evidence for aversive drug properties. We reinterpreted these data and hypothesized that rats avoid intake of the saccharin cue, at least in part, because the taste cue pales in perceived value relative to the potent drug of abuse expected in the very near future (Grigson, 1997). While the paradigm is more complex than originally thought (see below), evidence is available in support of this devaluation hypothesis in rats (see (Grigson, 2008) for a review) and in man. For example, in humans, the caudate has been shown, using fMRI, to be less responsive to losses or gains in monetary reward when the subject is waiting to smoke (Wilson et al., submitted).
In addition to drug-induced devaluation of the natural reward cue, however, we also have accumulated evidence suggesting that rats avoid the drug-paired gustatory cue because it comes to elicit the onset of a conditioned aversive state involving craving and withdrawal. Thus, like conditioned withdrawal (Coffey et al., 2013; Hotsenpiller, Giorgetti, & Wolf, 2001; McDonald, Parker, & Siegel, 1997), avoidance of the taste cue also is accompanied by elevated levels of circulating corticosterone (Gomez, Leo, & Grigson, 2000), blunted (Grigson & Hajnal, 2007) or even reversed (i.e., reduced below baseline) (Wheeler et al., 2011) levels of accumbens dopamine, and the onset of aversive taste reactivity (TR) behavior (e.g., gapes) following the intraoral delivery of the drug-paired taste cue (Wheeler et al., 2008). Importantly, greater avoidance of the taste cue (Grigson & Twining, 2002; Twining, Bolan, & Grigson, 2009), and a greater reduction in accumbens dopamine (Wheeler et al., 2011), reliably predict greater drug taking in rats. This makes sense, as drug-taking would serve as the most effective ‘correction’ for cue-induced withdrawal.
Responsiveness to the natural reward cue, then, provides information about who will take drug, when, and how much. In all cases described, save one (Grigson & Hajnal, 2007), both the rats and the humans have been well experienced with drug. This is true for the assessment of aversive taste reactivity behavior (i.e., gapes). Thus, in our previous experiment (Wheeler et al., 2008), the intraoral (IO) delivery (once/min/30 min) of one taste cue (referred to as the CS+) was paired with the opportunity to self-administer cocaine (0.33 mg/inf) for 2 h, and, on alternating days, the IO infusion of another taste cue (the CS−) was paired with the opportunity to self-administer saline for 2 h. Taste reactivity behavior was measured at the end of training and showed that the IO infusion of the CS+, but not the CS−, elicited strong aversive TR behavior (i.e., gapes). Moreover, a higher frequency of gapes elicited by the CS+ (i.e., greater aversion) was associated with a shorter end of study latency to take drug, greater within-session cocaine load-up behavior, and faster acquisition of steady state responding for cocaine. Importantly, in this 2008 study, as alluded to, all TR behavior was measured only at the end of training, after 8 to 15 taste-drug pairings. In a more recent manuscript (Wheeler et al., 2011), aversive TR was assessed after 5 pairings. In the present report, however, we did not simply want to correlate aversive taste reactivity behavior with drug self-administration behavior after the fact. To the contrary, we examined aversive taste reactivity behavior on a trial by trial basis beginning with the first cue-drug pairing. The goal here was to determine whether early changes in this conditioned behavior could predict later drug-seeking and drug-taking. Identification of an early indicator of vulnerability will allow for a better assessment of the development of the process of addiction in real time and will reveal a window for early intervention.
Methods
Subjects
The subjects were 21 adult male Sprague-Dawley rats obtained from Charles River (Wilmington, MA). They weighed between 304 and 612 grams at the beginning of the experiment. The first replication included 13 rats; the second replication included 8. They were housed individually in standard, metal cages in a temperature-controlled (21 °C) animal care facility with a 12/12 hour light/dark cycle (lights on at 7:00 am). All experimental manipulations were conducted during the light phase of the cycle. The rats were maintained with free access to water and to dry Purina rodent diet 5001, except where noted otherwise.
Surgery
Self-administration catheter
Intra-jugular catheters were custom-made in our laboratory as described previously (Puhl, Blum, Acosta-Torres, & Grigson, 2012; Puhl, Cason, Wojnicki, Corwin, & Grigson, 2011; Twining et al., 2009). General maintenance of catheter patency involved daily examination and flushing of catheters with heparinized saline. Catheter patency was verified as necessary using 0.2 ml of propofol (Diprivan 1%) administered intravenously.
Intraoral cannulae
PE 100 tubing (Intramedic, Sparks, MD) was cut into 8-cm segments, phalanged at 1 end, and fitted with a nylon washer (Grill & Norgren, 1978a, 1978b; Wheeler et al., 2011; Wheeler et al., 2008). Cannulae were maintained by daily examination and flushing with distilled water.
Procedure
In preparation for surgery, rats were anesthetized with intramuscular (IM) ketamine (70 mg/kg) and xylazine (14 mg/kg). Each rat received 300,000 units of subcutaneous (SC) Gpenicillin at the beginning of the surgical procedure, and 10 ml SC saline when complete. For each rat, a catheter was implanted in the right external jugular vein as described. The IO cannulae were implanted bilaterally as described (Grill & Norgren, 1978a, 1978b; Wheeler et al., 2011; Wheeler et al., 2008). Specifically, the tubing was inserted subcutaneously lateral to the 1st maxillary molar and secured with a nylon washer (.065 ID, .187 OD, .015 T, Product Components Corp.) between the molar and the cheek. The cannula was then secured at the excision point on the top of the head with a PTFE (Small Parts) washer, VetBond (3M Animal Care), and superglue (Loctite 409). When righting reflexes had recovered, rats were returned to their home cages with mash and solid chow available ad lib. Rats recovered for 17-20 days (first replication) or 33 days (second replication) before habituation began. During this time, solid chow and water were available ad lib, and nutritional support (e.g., SC fluids) was provided as needed.
Apparatus
Each rat was trained in one of 4 identical operant chambers (MED Associates, St. Albans, VT). Each chamber measures 29.3 cm in length × 24.0 cm in width × 27.0 cm in height, and is individually housed in a light- and sound-attenuated cubicle. The floor and back wall are clear Plexiglas; the side walls are aluminum. Each chamber is equipped with three retractable sipper spouts that enter through 1.3-cm diameter holes, spaced 16.4 cm apart (center to center). A stimulus light is located 6.0 cm above each tube. Each chamber also is equipped with a houselight (25 W), a tone generator (Sonalert Time Generator, 2900 Hz, Mallory, Indianapolis, IN), and a speaker for white noise (75 dB). Cocaine reinforcement is controlled by an infrared motion detector circuit that monitors nosepokes on an empty spout to operate a syringe pump (Model PHM-100VS, MED Associates, St. Albans, VT). A coupling assembly attaches the syringe pump to the catheter assembly on the back of each rat and enters through a 5.0-cm diameter hole in the top of the chamber. This assembly consists of a metal spring attached to a metal spacer with Tygon tubing inserted down the center, protecting passage of the tubing from rat interference. The tubing is attached to a 5-channel counterbalanced swivel assembly (Instech, Plymouth Meeting, PA) that, in turn, is attached to the syringe pump. An angled mirror is located below the floor, allowing for a view of the ventral surface of the rat. Video is collected with a high-speed camera (100 frames/sec) positioned below the floor of the chamber that recorded orofacial responses following intraoral infusion of the gustatory stimuli (Basler). For the first replication, lighting for video was provided by 4 fluorescent lights (2 at 9W and 2 at 8W) located below the chamber. For the second replication, lighting for video was provided by 2 fluorescent lights (each 8W) located below the chamber, and a green panel light (0 – 500 lux; CleverSys, Inc., Reston, VA) that served as the chamber’s ceiling. Events in the chamber and collection of data were controlled on-line with a Pentium computer that used programs written in the Medstate notation language (MED Associates).
Conditioning procedures
Water training
Ad lib water was removed 9 hours into the light phase of the last recovery day. For the next eight days, rats were trained to drink from a bottle at the front of the home cage. Distilled water was available for 15 minutes each morning, beginning 2 hours into the light phase and for 1 hour each afternoon, beginning 6 hours into the light phase.
Habituation
Thereafter, beginning 2 hours into the light phase, rats received one 10-minute chamber habituation session daily for 3 days. During this 10-min period, water was available, with the location of the spout varied across location 1-3 (left, middle, right). Each day rats were give overnight access to 20 ml distilled water at the front of the home cage, beginning about 75 minutes after being returned from the chamber to their home cage. This water restriction regimen continued throughout testing.
Cocaine self-administration
Using a within subjects design, self-administration sessions consisted of a 30-minute IO infusion period during which IO infusions of the tastant (CS+ or CS−, 0.186% grape or orange unsweetened Kool-Aid in 0.15% saccharin, with flavors counterbalanced between rats) were delivered at a rate of 1 infusion (0.2 ml delivered over 3.5 s) per minute for 30 min. Thereafter, the middle and right empty spouts advanced and completion of a fixed number (FR1 on Trials 1 – 4; FR5 on Trials 5 – 16; FR10 on Trials 17 – 30) of contacts on the active (right) empty spout led to an iv infusion of saline on CS− trials or 0.33 mg/infusion cocaine on CS+ trials for a 90 min (replication 1) or a 120 min (replication 2) access period. Each infusion was followed by a 20-sec time out period, during which the cue light was turned off, the house light was illuminated, the spouts were retracted, and a tone sounded. Contacts on the inactive middle spout were recorded, but were without consequence. There were a total of 15 such CS+ trials and 15 CS− trials (see Fig.1).
Figure 1.

Sequence of trials during the experiment.
In most cases (i.e., 17/21), training began with a CS+ trial, as this order supported faster acquisition. Video was recorded only during intraoral infusion periods throughout the experiment. Therefore, the chamber lights were on during the I/O period, but were manually turned off at the beginning of the self-administration period (the bright lights appeared to inhibit responding). Rats had overnight access to 20 ml of distilled water at the front of the home cage beginning about 45 minutes after being returned from the operant chamber.
Data analysis
At the end of the study, gapes were scored manually by a reviewer blind to rat identity and tastant. Dependent measures included: the number of gapes emitted to the CSs, the latency to the first response on the active and the inactive spout, the number of responses emitted on the active and inactive spout, the latency to the first infusion, the number of load-up infusions, and the total number of infusions/session. A log10 transformation was used for the latency data to minimize the impact of a few extreme scores. Load-up was operationally defined as the number of infusions taken at interinfusion intervals less than the mean interinfusion interval for the session. Thereafter, the data for each trial were analyzed using 2 × 15 mixed factorial analysis of variance (ANOVAs) varying group (high vs. low drug-takers) and trial (1-15) and, where relevant, a 2 × 30 mixed factorial ANOVA varying group and CS infusion period (1-30).
The Group × Trials interactions rarely attainted statistical significance, likely because differences between low and high drug-takers emerged early and were sustained across trials. As a consequence, only significant interactions are described below, followed by post hoc Student Newman-Keuls tests with p < .05. Further, because the primary objective of this study was to determine the point of emergence of conditioned aversive taste reactivity behavior to the drug-paired CS+ solution, planned t-tests were conducted on a trial by trial basis for each measure between the high and low drug-takers. Statistical procedures were performed in Statistica7 (StatSoft) and SPSS 20 (IBM). Drug self-administration behavior will be discussed first, followed by taste reactivity behavior emitted following IO delivery of the gustatory cue.
Results
Six rats were excluded from the first replication and 3 were excluded from the second replication because of health issues or loss of iv catheter or IO cannula patency. The final number of rats included in the analyses was 7 from replication 1 and 5 from replication 2, for a total of 12.
Terminal cocaine infusions
As shown in Fig. 2, rats were divided into 2 groups, high and low drug-takers, based upon the number of cocaine infusions taken during terminal CS+ trials 14 and 15. As has been reported previously, these subjects fell into distinct groups, with high drug-takers clearly taking significantly more cocaine during the last 2 taste-drug pairings than low drug-takers (13.25±4.25 vs. 0.58±.08 infusions per session, F2,9 = 8.74; p < .01).
Figure 2.
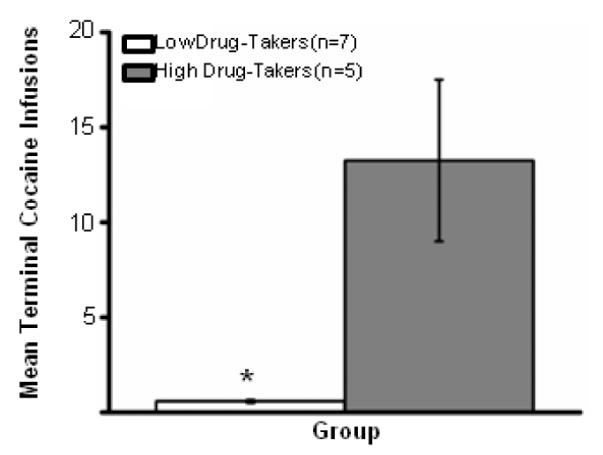
Mean Number of Terminal Cocaine Infusions (averaged across CS+ trials 14 and 15) self-administered by Low (n=7) and High (n=5) drug-takers. *, p < .05.
Cocaine Self-Administration Behavior
Having segregated rats by the number of infusions taken at the end of the study, we compared their other operant behaviors in an effort to fully characterize the behavior of these two groups of subjects. As described, following the overall ANOVA, responding by the high and low drug-takers was compared on a trial by trial basis using planned t-tests to determine the point at which differences in behavior emerged. Figure 3 summarizes data gleaned from CS+ trials.
Figure 3.
Log10 latency in sec to make the first lick (panels A and B), mean number of active spout responses (panels C and D), Log10 latency in sec to the first cocaine infusion (panels E and F), Load-up infusions (panels G and H), and total number of infusions self-administered (panels I and J) as a function of the Low and High drug-takers overall (left panels) and for Low and High drug-takers across 15 CS+ trials. *, p <.05; #, p <.01; †, p < .001.
Latency to First Response on the Active Spout
A repeated-measures ANOVA of the log10 response latencies revealed a significant main effect of group, with high drug-takers initiating contact with the active spout more quickly than low drug-takers, overall, F10,1= 6.67; p < .05; Fig. 3A. The results of independent sample t-tests at each trial indicated that high drug-takers initiated licking on the active spout with a significantly shorter latency than low drug-takers on Trial 8, t (10) = 2.38, p < .05; Fig. 3B.
Number of Responses on Active Spout
A repeated-measures ANOVA of the responses on the active spout indicated a significant main effect of group, F10,1= 71.60; p < .001. Thus, as shown in Fig. 3C, high drug-takers made more responses on the active spout, overall, than did low drug-takers. The main effect of trial also was significant, F140,14=1.79; p < .05, indicating that the number of active responses increased across trials. According to the results of independent sample t-tests, the high drug-takers emitted significantly more responses on the active spout than the low drug-takers, beginning with Trial 5, ps<.05 (Fig. 3D).
Latency to First Cocaine Infusion
A repeated-measures ANOVA of the infusion log10 latency revealed significant main effects of group, F10,1 = 11.96, p < .01; Fig. 3E, and trial, F140,14 = 2.94; p <.001. These results showed that high drug-takers initiated their first infusion earlier in the session than the low drug-takers. The Group × Trial interaction did not attain statistical significance, F < 1. Comparison of the means on each trial (Fig. 3F) using planned independent sample t-tests indicated that high drug-takers initiated their first infusion of cocaine significantly earlier than low drug-takers on trials 8, t10=4.05, p = .01; 9, t10 =2.43, p < .05; 13, t10 =4.85; p <.01; and 14, t10 = 5.16, p <.001.
Number of Cocaine Load-Up Infusions
As shown in Fig. 3G, high drug-takers also engaged in higher levels of cocaine self-administration early in the session (i.e., they exhibited greater load-up behavior) than did the low drug-takers. Support for this conclusion is provided by a significant main effect of Group (F10,1 = 6.20; p = .03). There also was a significant main effect of Trial and a significant Group × Trial interaction (F140, 14 = 2.29, p < .01). A similar pattern was evidenced on Trials 8 and 9 with follow-up independent sample t-tests (ps < .05).
Total Number of Cocaine Infusions
Finally, a repeated-measures ANOVA of the total number of cocaine infusions taken per trial across the 15 trials (Fig. 3I) revealed a significant main effect of Group (F10,1 = 15.86, p < .003), with high drug-takers self-administering more cocaine than low drug-takers over the course of the study overall. The main effect of Trial (F140, 14 = 1.5, p = .12) and the Group × Trial interaction (F140, 14 = 1.05, p = .40) did not attain significance. Analysis of the individual trials using students t-tests indicated that high drug-takers took significantly more cocaine infusions than low drug-takers on trial 2 and on trials 6 - 15, ps < .05 (Fig. 3J). Importantly, while the high drug-takers took more drug than the low drug-takers on several trials (see Fig. 3J), correlational analyses revealed that the number of cocaine infusions self-administered early in training (i.e., on trials 1 – 8) failed to predict terminal (i.e., mean of last 2 CS+ trials) cocaine infusions (ps >.05).
Saline Self-Administration Behavior
To test whether behavioral differences between high and low drug-takers for cocaine carried over to saline in this within subjects design, we examined performance by these same subjects when responding for saline on CS− trials (data not shown). Unlike responding for cocaine, the results revealed that neither the main effect of group nor the Group × Trials interaction was significant when considering the log10 latency to make the first active spout response for a saline infusion, the number of active spout responses emitted, the log10 latency to elicit the first saline infusion, and the total number of saline infusions elicited, ps > 0.05. That said, students t-tests of the Group × Trials interactions did show that high drug-takers initiated responding for saline more quickly than low drug takers on at least one trial and took more saline infusions on two trials, ps < .05. Even so, when averaged across trials, responding for saline was quite low (High Drug-Takers: 5.15±2.49; Low Drug-Takers: 1.36±0.37). Group differences in active spout responding, then, were fairly selectively driven by cocaine.
Taste Reactivity
According to Wheeler et al.(2008) , aversive taste reactivity to the CS+, but not to the CS−, following 8-15 taste-drug pairings corresponded to a shorter latency to take drug, increased load-up, and an increase in the total number cocaine infusions. In contrast to the cited study, which assessed taste reactivity only at the end of training, here we measured aversive taste reactivity behavior (gapes; Fig. 4A) on every trial in an effort to monitor how a rat’s evaluation of the tastants changed with experience and whether early changes in such behavior could be used to predict later drug-seeking and drug-taking.
Figure 4.
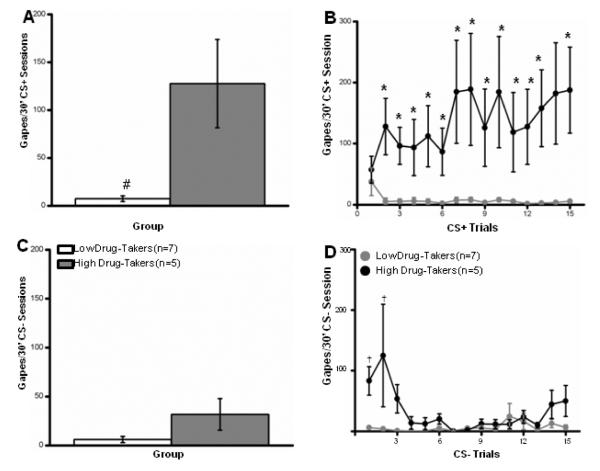
Panels A and B. Mean (+/− SEM) number of gapes emitted/30 min CS+ sessions for Low and High Drug-Takers overall (A) and for Low and High Drug-Takers across 15 CS+ trials. Panels C and D. Mean (+/− SEM) number of gapes emitted/30 min CS− sessions for Low and High Drug-Takers overall (A) and for Low and High Drug-Takers across 15 CS− trials. *, p < .05; #, p <.01; †, p < .001.
Across-Trial Analysis of Aversive Taste Reactivity
The number of gapes emitted by low and high drug-takers was assessed across CS+ or CS− trials using separate 2 × 15 mixed factorial ANOVAs varying group (low vs. high) and trials (1-15). The results found a significant main effect of Group for gapes to the CS+ (F10,1 = 9.55, p < .01), Fig. 4A, but not to the CS− (F10,1 = 3.34, p = .10); Fig. 4C. The Group × Trial interaction was not significant for responding during the CS+ condition (F140,14 = 1.27, p = .24). With the exception of Trial 14, however, the results of the 1-way ANOVAs revealed that high drug-takers made more gapes to the CS+ solution than did low drug-takers following a single CS+-cocaine pairing, ps < .05 (Fig. 4B). For the CS− data, a significant main effect of Trial was obtained (F140,14 = 3.14, p < .001), indicating that the number of gapes to the CS− decreased across trials. Post-hoc analysis of a significant Group × Trial interaction (F140,14 = 3.26, p < .001) showed that high drug-takers gaped more than low drug-takers to the saline-associated taste cue, but only on CS− Trials 1, p < .01, and 2, p < .001, (Fig. 4D). Given that training began with the CS+-cocaine trial for 9/12 subjects, the occurrence of gapes upon the first CS− trail, is interpreted as early generalization from the CS+ to the CS−.
Within-Trial Analysis of Aversive Taste Reactivity
The temporal distribution of gaping behavior averaged across all fifteen 30-min I/O infusion sessions was then examined separately for the CS+ and the CS− conditions using 2 × 30 mixed factorial ANOVAs varying group (high vs. low drug-takers) and IO infusions (1 – 30) as factors. For our analysis, gapes that occurred after Infusion 1 ended, but before Infusion 2 began, were included in the Infusion 1 count. As shown in Fig. 5A, and confirmed by a significant main effect of group (F24,1 = 79.2, p < .001), the high drug-takers made more gapes following the intraoral delivery of the CS+ solution than did the low drug-takers across the 30 min CS+ infusions session, overall. The main effect of infusion (F696,29 = 4.4, p < .001) and the Group × Infusion interaction (F696,29 = 1.7, p < .001) were also significant. Follow-up t-tests revealed that this difference was significant for each of 30 infusions, with the greatest number of gapes being emitted during infusions 6-10 compared to infusions 1-5 (p < .05), 16-20 (p <.001), 21-25 (p <.001), and 26-30 (ps < .01) (trials 6-10 are indicated by a bracket in Fig. 5A).
Figure 5.
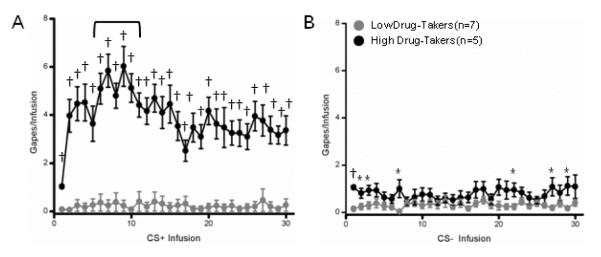
Mean (+/− SEM) number of gapes emitted/IO infusion for Low and High Drug-Takers across 30 CS+ (Panel A) or CS− (Panel B) infusions. *, p < .05; #, p <.01; †, p < .001.
Like responding for the CS+ solution, the number of gapes emitted following the CS− infusion also differed between the low and the high drug-takers (F24,1 = 7.2, p < .01). However, the main effect of infusion (F < 1) and the Group × Infusion interaction (F < 1) were non-significant. Even so, in an effort to be consistent with the analysis of the CS+ data, follow-up t-tests were conducted to compare the number of gapes emitted by the low and high drug-takers on each infusion. The results showed that High Drug-Takers gaped more than Low Drug-Takers during CS− IO infusions 1-3, 7, 22, 27, and 29 (Fig. 5B). That said, and while this is evidence of some generalization between the CS+ and the CS− solution, the high drug-takers clearly exhibited far more gapes during the intraoral infusion of the CS+ (Fig 5A) than during the intraoral infusion of the CS− (Fig 5B).
Early aversive taste reactivity predicts cocaine-taking
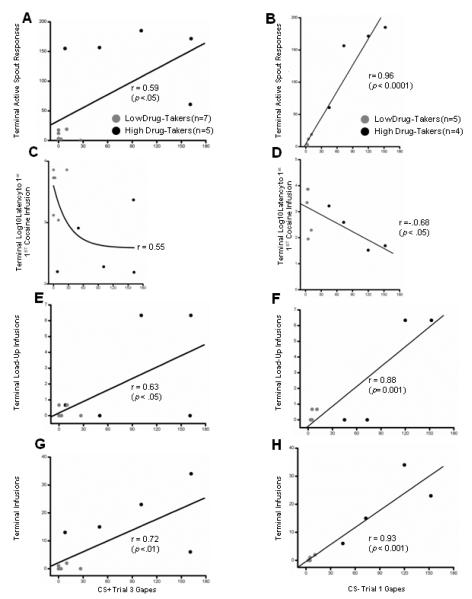
Figure 6 summarizes the correlational data between aversive TR to the CS+ (left panels) or the CS− (right panels) early in the study with drug-related behavior at the end of the study. Neither Trial 1 nor Trial 2 gapes to the CS+ reliably predicted drug-taking behavior. The following significant correlations were found between Trial 3 gapes to the CS+ (Fig 6, left panels) and Trial 1 gapes to the CS− (Fig 6, right panels), and terminal drug-taking behaviors using a 1-tailed Pearson’s correlation coefficient. Terminal drug-taking was determined, as described, by averaging performance across the last 2 CS+ or CS− trials.
Figure 6.
Gapes emitted to the CS+ on Trial 3 (left panels) and to the CS− on Trial 1 (right panels) for the Low and High Drug-Takers as a function of terminal active spout responses (Panels A and B), Terminal Log10 Latency to make the first cocaine infusion (Panels C and D), Terminal Load-UP Infusions (Panels E and F), and Terminal Infusions (Panels G and H) during Trials 14 and 15.
Results showed that greater Trial 3 gapes to the CS+ predicted greater terminal active spout responses, r = .59, p < .05 (Fig. 6A), greater terminal load-up infusions, r = .63, p < .05 (Fig. 6E), and a greater number of total terminal infusions, r = .72, p < .01, (Fig 6G). Trial 1 gapes to the CS− (including data only from those rats for whom a CS+ trial preceded the first CS− trial) predicted number of terminal active spout responses, r = .96, p < .0001, (Fig. 6B), terminal log10 latency to first infusion, r = −.68; p < .05, (Fig 6D), terminal load-up infusions, r = .88; p <.001, (Fig. 6F), and terminal infusions, r = .93, p < .001, (Fig 6H). Finally, gapes emitted to the CS+ on Trial 3 were highly correlated with the number of gapes emitted to the CS− on Trial 1, r = .75, p < .003 (data not shown).
Discussion
In the present study, we report that rats who ultimately became high drug-takers emitted greater aversive taste reactivity behavior (i.e., gaped more frequently) following a single taste-drug pairing than did rats that ultimately became low drug-takers. Moreover, correlational analyses show that those rats that exhibited the most aversive TR upon their third exposure to the drug-paired taste cue exhibited the greatest responsiveness to drug as assessed during the last 2 self-administration trials. Specifically, gapes to the CS+ following 2 taste-drug pairings (i.e., on the 3rd CS+ trial) reliably predicted operant responding for drug, load-up infusions, and total cocaine infusions in the last 2 cocaine self-administration periods. Likewise, the first intraoral infusion of the CS− elicited a similar pattern of aversive TR behavior and this was highly predictive of later drug seeking and taking. Aversive taste reactivity to a drug-paired cue, then (via direct pairing or via generalization to the explicit drug-paired cue), occurs very early in training and, importantly, predicts later drug-seeking and drug-taking.
So, why is the drug-paired taste cue eliciting aversive taste-reactivity behavior in the present experiment? Rats prefer a context that has been paired with cocaine and they readily self-administer the drug (Ahmed & Koob, 1998; Pettit, Ettenberg, Bloom, & Koob, 1984). Cues coincidentally paired with drugs of abuse elicit approach behaviors and they support a conditioned increase in levels of dopamine in the nucleus accumbens (Weiss et al., 2001; Wheeler et al., 2011). Finally, the intraoral delivery of a taste cue paired closely in time with experimenter administered cocaine, phencyclidine, or methamphetamine elicits little to no aversive TR (Parker, 1993). In an effort to square this circle, we look to the literature and, in particular, to one recent report. Specifically, it was recently shown that a drug-paired taste cue (like that employed here) can elicit either conditioned reward or conditioned aversion, depending upon when the cue is delivered relative to drug infusion (Wheeler et al., 2011). Thus, in agreement with the above mentioned literature showing evidence for conditioned reward, the intraoral infusion of a gustatory CS that had previously been paired with the simultaneous iv infusion of self-administered cocaine elicited evidence of a positive state involving ingestive, rather than aversive, taste reactivity behavior (i.e., licks rather than gapes) and a conditioned increase in dopamine in the nucleus accumbens. On the other hand, the very same gustatory cue elicited the onset of a negative affective state marked by aversive TR, reduced dopamine levels in the nucleus accumbens, and an elevated threshold for intracranial self-stimulation when the taste cue was infused once/min for 45 min prior to the opportunity to self-administer cocaine. This latter procedure is that which was employed here. Taken together, the data indicate that conditioned aversion does not depend upon rewarding and/or aversive properties of the drug (Arthurs, Lin, Amodeo, & Reilly, 2012) per se, or upon a subject’s relative sensitivity to those properties(Verendeev & Riley, 2012), because the drug in the Wheeler et al. manuscript (2011) was the same whether presented coincident with the gustatory cue or following presentation of the gustatory cue. Rather, the data suggest, and we conclude, that conditioned aversion depends upon the nature of the predictive relationship between the cue and the consequence and the underlying physiology that supports that relationship.
As alluded to in the Introduction, the conditioned aversive state elicited by cued anticipation of drug availability may reflect the development of an aversive opponent process involving conditioned withdrawal. As such, the CS+ may serve as an occasion setter or discriminative stimulus that elicits conditioned compensatory responses in anticipation of drug (Ramos, Siegel, & Bueno, 2002; Siegel, 2001). In accordance, like drug-induced avoidance of the gustatory cue, cue-induced withdrawal also is accompanied by elevated corticosterone (Gomez et al., 2000), blunted dopamine in the nucleus accumbens (Hotsenpiller, et al., 2001; Wheeleret al., 2011), and aversive TR behavior following the intraoral delivery of a naloxone-paired saccharin cue (Nyland & Grigson, 2013). Thus, while drug-seeking and taking may be promoted by positive reinforcement, it also is promoted by negative reinforcement, as only drug serves to correct this conditioned aversive state (Solomon & Corbit, 1973, 1974; Wheeler et al., 2011). In accordance, in rats, greater conditioned avoidance/aversion is associated with greater drug-seeking, greater load-up behavior, and greater drug-taking (Wheeler et al., 2011; Wheeler et al., 2008). In humans, negative affect also is evident when waiting for access to nicotine in the presence of drug-related cues(Sayette et al., 2003) and a failure to respond to monetary rewards, as indicated by blunted fMRI activity of the nucleus accumbens, predicts a short latency to choose a cigarette over money in a subsequent choice test (Wilson et al., submitted).
Our data indicate that for rats who became high drug-takers, the CS+ acquired significant occasion-setting properties after only one pairing with cocaine. This seems to fit well with evidence that a single taste-morphine pairing was sufficient to produce conditioned avoidance of the drug-paired taste cue and a conditioned blunting of the accumbens dopamine response to the otherwise palatable saccharin cue in rats (Grigson & Hajnal, 2007). It also has been shown that a single pairing of environmental cues with cocaine self-administration is sufficient to produce CS induced reinstatement of cocaine-seeking behavior when rats are exposed to a 1 h extinction test 9 months later (Ciccocioppo, Martin-Fardon, & Weiss, 2004). Finally, like rats, adolescent humans can reportedly lose control over tobacco (i.e., exhibit symptoms of dependence) within a day or 2 of their first inhalation (DiFranza et al., 2007) and even one symptom of dependence strongly predicts continued use (DiFranza et al., 2002).
We would argue, then, that addiction can begin with the first exposure to drug and that this animal model provides not only a window on the development of this process, but a clear and very early indicator of individual vulnerability to, and resilience from, addiction. Thus, some drug-experienced rats exhibit greater avoidance of the taste cue, greater aversive TR behavior following the IO infusion of the drug-paired cue, greater conditioned elevation of corticosterone, and when challenged with naloxone, greater evidence for conditioned withdrawal ((Gomez et al., 2000; Nyland & Grigson, 2013; Wheeler et al., 2008); see Fig 5). Where tested, these same rats then exhibit the shortest latency to take drug, the greatest load-up behavior, and the greatest seeking and taking of drug (Grigson & Twining, 2002; Nyland & Grigson, 2013). Here, in our hands, aversive TR was found to occur very early and to predict later drug-seeking and drug-taking. Future studies will need to determine whether vulnerable individuals exhibit exaggerated conditioned responses early on because (1) the drug has a greater physiological impact upon first infusion in these subjects, (2) the palatable taste cue mitigates the impact of drug for some and less for others, or (3) a heightened regulatory process allows for the development of stronger conditioning of the opponent process (experienced as cue-induced withdrawal). Finally, we have identified a number of factors that affect avoidance of a drug-paired cue and/or responding for drug. For example, female Sprague-Dawley rats exhibit more acceptance of a cocaine-paired saccharin cue and less cocaine self-administration in the taste-drug model than male rats (Cason & Grigson, 2013). Environmental enrichment during adulthood reduces acquisition of cocaine self-administration in male rats (Puhl et al., 2012). Chronic sleep deprivation, on the other hand, greatly augments acquisition of cocaine self-administration and the willingness to work for drug (Puhl, Boisvert, Guan, Fang, & Grigson, 2013; Puhl, Fang, & Grigson, 2009), as does a history of having binged on fat (Puhl et al., 2011). Thus, having identified an early indicator of vulnerability (i.e., conditioned aversive taste reactivity behavior to the drug-paired cue), future studies also will seek to intervene, behaviorally and/or pharmacologically, to prevent this early transition from use to abuse and addiction.
Acknowledgements
We thank Sarah Ballard for her assistance with data collection and the National Institute on Drug Abuse for generously providing the cocaine HCl for this study. This research was supported by NIH grant DA009815.
Footnotes
The authors declare no competing financial interests.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed Author; Washington, DC: 2000. [Google Scholar]
- Arthurs J, Lin JY, Amodeo LR, Reilly S. Reduced Palatability in Drug-Induced Taste Aversion: II. Aversive and Rewarding Unconditioned Stimuli. Behav Neurosci. 2012 doi: 10.1037/a0027676. doi: 10.1037/a0027676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, LeBlanc AE. Conditioned aversion to saccharin by single administrations of mescaline and d-amphetamine. Psychopharmacologia. 1971;22(4):352–356. doi: 10.1007/BF00406873. [DOI] [PubMed] [Google Scholar]
- Cason AM, Grigson PS. Prior access to a sweet is more protective against cocaine self-administration in female rats than in male rats. Physiol Behav. 2013;112-113:96–103. doi: 10.1016/j.physbeh.2013.02.017. doi: 10.1016/j.physbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nature Neuroscience. 2004;7(5):495–496. doi: 10.1038/nn1219. doi: 10.1038/nn1219. [DOI] [PubMed] [Google Scholar]
- Coffey KR, Barker DJ, Ma S, Root DH, Martinez L, Horvitz JC, West MO. Effects of varying reinforcement probability on pavlovian approach behavior and ultrasonic vocalizations in rats. Behav Brain Res. 2013;237:256–262. doi: 10.1016/j.bbr.2012.09.041. doi: 10.1016/j.bbr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CM, Riley AL. Conditioned taste aversion learning: implications for animal models of drug abuse. Ann N Y Acad Sci. 2010;1187:247–275. doi: 10.1111/j.1749-6632.2009.05147.x. doi: 10.1111/j.1749-6632.2009.05147.x. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, O’Loughlin J, Pbert L, Ockene JK, Wellman RJ. Symptoms of tobacco dependence after brief intermittent use: the Development and Assessment of Nicotine Dependence in Youth-2 study. Arch Pediatr Adolesc Med. 2007;161(7):704–710. doi: 10.1001/archpedi.161.7.704. doi: 10.1001/archpedi.161.7.704. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, Wood C. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob Control. 2002;11(3):228–235. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, Volkow ND. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug and Alcohol Dependence. 2007;87(2-3):233–240. doi: 10.1016/j.drugalcdep.2006.08.022. doi: 10.1016/j.drugalcdep.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez F, Leo NA, Grigson PS. Morphine-induced suppression of saccharin intake is correlated with elevated corticosterone levels. Brain Research. 2000;863:52–58. doi: 10.1016/s0006-8993(00)02093-x. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: a reinterpretation. Behavioral Neuroscience. 1997;111(1):129–136. [PubMed] [Google Scholar]
- Grigson PS. Reward comparison: the Achilles’ heel and hope for addiction. Drug Discov Today Dis Models. 2008;5(4):227–233. doi: 10.1016/j.ddmod.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Hajnal A. Once is too much: conditioned changes in accumbens dopamine following a single saccharin-morphine pairing. Behav Neurosci. 2007;121(6):1234–1242. doi: 10.1037/0735-7044.121.6.1234. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behav Neurosci. 2002;116(2):321–333. [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978a;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Research. 1978b;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. European Journal of Neuroscience. 2001;14(11):1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Jones S, Casswell S, Zhang JF. The Economic Costs of Alcohol-Related Absenteeism and Reduced Productivity among the Working Population of New-Zealand. Addiction. 1995;90(11):1455–1461. doi: 10.1046/j.1360-0443.1995.901114553.x. doi: 10.1046/j.1360-0443.1995.901114553.x. [DOI] [PubMed] [Google Scholar]
- Le Magnen J. Peripheral and systemic actions of food in the caloric regulation of intake. Annals of the New York Academy of Sciences. 1969;157(2):1126–1157. doi: 10.1111/j.1749-6632.1969.tb12940.x. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proc Assoc Am Physicians. 1999;111(2):99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Lester D, Nachman M, Le Magnen J. Aversive conditioning by ethanol in the rat. Q J Stud Alcohol. 1970;31(3):578–586. [PubMed] [Google Scholar]
- McDonald RV, Parker LA, Siegel S. Conditioned sucrose aversions produced by naloxone-precipitated withdrawal from acutely administered morphine. Pharmacology Biochemisty and Behavior. 1997;58(4):1003–1008. doi: 10.1016/s0091-3057(97)00313-4. [DOI] [PubMed] [Google Scholar]
- Nair P, Black MM, Schuler M, Keane V, Snow L, Rigney BA, Magder L. Risk factors for disruption in primary caregiving among infants of substance abusing women. Child Abuse Negl. 1997;21(11):1039–1051. doi: 10.1016/s0145-2134(97)00064-1. doi: 10.1016/S0145-2134(97)00064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse Drug Abuse and Addiction: One of America’s Most Challenging Public Health Problems. from http://archives.drugabuse.gov/about/welcome/aboutdrugabuse/magnitude/
- Nyland JE, Grigson PS. A drug-paired taste cue elicits withdrawal and predicts cocaine self-administration. Behav Brain Res. 2013;240:87–90. doi: 10.1016/j.bbr.2012.10.057. doi: 10.1016/j.bbr.2012.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA. Taste reactivity responses elicited by cocaine-, phencyclidine-, and methamphetamine-paired sucrose solutions. Behav Neurosci. 1993;107(1):118–129. doi: 10.1037//0735-7044.107.1.118. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 1984;84(2):167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Puhl MD, Blum JS, Acosta-Torres S, Grigson PS. Environmental enrichment protects against the acquisition of cocaine self-administration in adult male rats, but does not eliminate avoidance of a drug-associated saccharin cue. Behav Pharmacol. 2012;23(1):43–53. doi: 10.1097/FBP.0b013e32834eb060. doi: 10.1097/FBP.0b013e32834eb060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Boisvert M, Guan Z, Fang J, Grigson PS. A novel model of chronic sleep restriction reveals an increase in the perceived incentive reward value of cocaine in high drug-taking rats. Pharmacol Biochem Behav. 2013 doi: 10.1016/j.pbb.2013.04.010. doi: 10.1016/j.pbb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Cason AM, Wojnicki FHE, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behavioral Neuroscience. 2011;121(6):930–942. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Fang J, Grigson PS. Acute sleep deprivation increases the rate and efficiency of cocaine self-administration, but not the perceived value of cocaine reward in rats. Pharmacol Biochem Behav. 2009;94(2):262–270. doi: 10.1016/j.pbb.2009.09.005. doi: 10.1016/j.pbb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BM, Siegel S, Bueno JL. Occasion setting and drug tolerance. Integr Physiol Behav Sci. 2002;37(3):165–177. doi: 10.1007/BF02734179. [DOI] [PubMed] [Google Scholar]
- Santolaria-Fernandez FJ, Gomez-Sirvent JL, Gonzalez-Reimers CE, Batista-Lopez JN, Jorge-Hernandez JA, Rodriguez-Moreno F, Hernandez-Garcia MT. Nutritional assessment of drug addicts. Drug Alcohol Depend. 1995;38(1):11–18. doi: 10.1016/0376-8716(94)01088-3. doi: 10.1016/0376-8716(94)01088-3. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Perrott MA, Hobel J. Effects of smoking opportunity on cue-elicited urge: a facial coding analysis. Exp Clin Psychopharmacol. 2003;11(3):218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. Pavlovian conditioning and drug overdose: when tolerance fails. Addiction Research and Theory. 2001;9(5):503–513. doi: 10.3109/16066350109141767. [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81(2):158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81(2):119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-44. Rockville, MD: 2012. (HHS Publication No. (SMA) 12-4713) [Google Scholar]
- Twining RC, Bolan M, Grigson PS. Yoked Delivery of Cocaine Is Aversive and Protects Against the Motivation for Drug in Rats. Behavioral Neuroscience. 2009;123(4):913–925. doi: 10.1037/a0016498. doi: 10.1037/A0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. Conditioned taste aversion and drugs of abuse: history and interpretation. Neurosci Biobehav Rev. 2012;36(10):2193–2205. doi: 10.1016/j.neubiorev.2012.08.004. doi: 10.1016/j.neubiorev.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben-Shahar O. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25(3):361–372. doi: 10.1016/S0893-133X(01)00238-X. doi: S0893 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57(5):774–785. doi: 10.1016/j.neuron.2008.01.024. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Delgado MR, Mckee SA, Grigson PS, MacLean RR, Nichols TT, Henry SL. Ventral striatal responses to monetary outcomes predict the ability to resist smoking. (submitted) [DOI] [PMC free article] [PubMed]