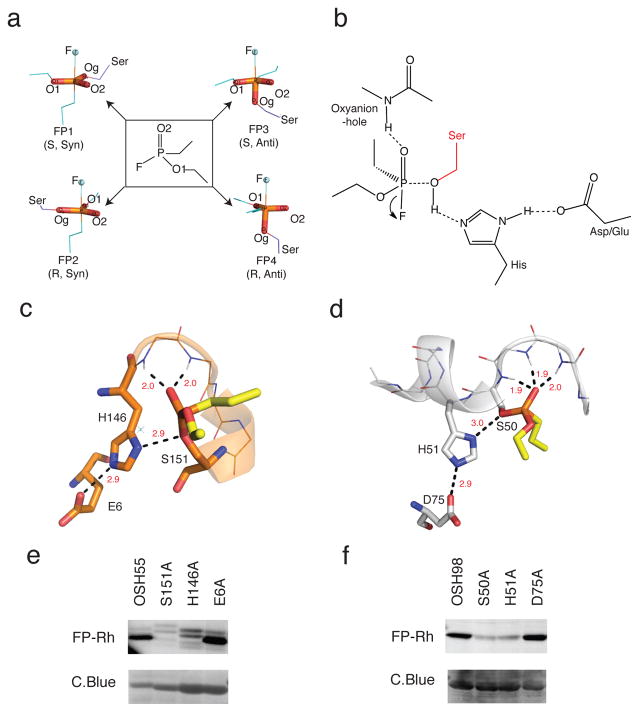
Figure 1. Design strategy.
(a) Transition-state (TS) models. The fluorophosphonate (FP) ligand (ethyl ethylphosphonofluoridate) used in design calculations is in the middle panel flanked by TS models corresponding to syn-and anti- attack on the two isomers (R & S) of the ligand with the leaving atom fluorine (F) in blue-spheres. The serine nucleophile is shown in purple. (b) Theozyme geometry. Geometric parameters were derived from the computed ideal active site geometries and native hydrolase statistics previously reported30. Theozymes with the histidine hydrogen bonding reversed were also considered during RosettaMatch (See Methods). (c.d) Design models of OSH55 (c) and OSH98 (d). Active-site residues of OSH55 and OSH98 are shown in orange and white sticks respectively. The modeled fluorophosphonate ligand is shown in yellow sticks. In both designs, multiple backbone NHs form the oxyanion hole to stabilize the transition state. (e,f) Selective FP-Rh labeling of OSH55 (e) and OSH98 (f) but not the active site serine knockouts. Serine reactivity was assessed by gel-based ABPP (top, in-gel fluorescence; bottom, coomassie staining, see Supplementary Fig. 15 for full-size gel images. The experiments were performed in duplicates with consistent results).