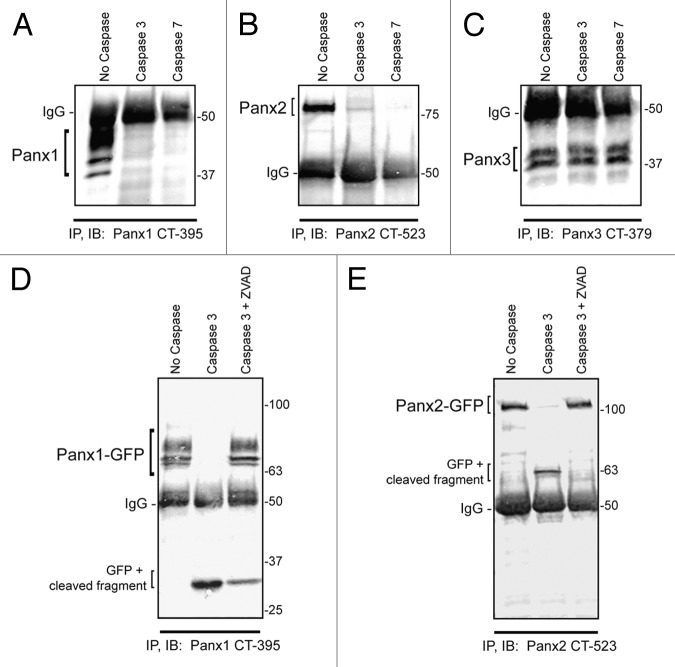
Figure 2. Panx1 and Panx2 are substrates for caspase cleavage. In vitro caspase 3 and caspase 7 cleavage of Panx1, -2, and -3, ectopically expressed in HEK-293T cells. Panx-CT antibodies were used for pull down (IP) of all 3 pannexins, treated with: buffer only, exogenous caspase 3 or 7 (500 nM), or both caspase 3 (500 nM) and the pan-caspase inhibitor Z-VAD-OMe-FMK (50 µM). After immunoblotting (IB) with Panx-CT antibodies, the absence of protein bands indicated caspase cleavage of Panx1 (A) and Panx2 (B) as compared with controls. The presence of Panx3 protein bands despite the addition of activated exogenous caspase suggested that Panx3 is not a substrate for caspase-dependent cleavage in vitro. The IgG (50 kDa) antibody heavy chain is found in all 3 blots as expected. Cleaved protein bands detected at ~32 kDa for Panx1-GFP (D) and ~63 kDa for Panx2-GFP (E) suggested that the caspase cleavage sites are located in the C-terminal domains of both proteins. Cleavage is inhibited in the presence of the pan-caspase inhibitor Z-VAD. Protein sizes are noted in kDa.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.