Figure 1.
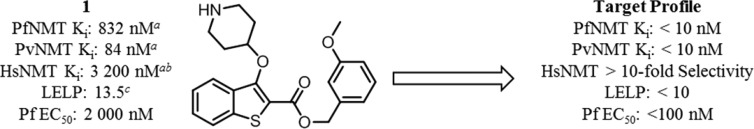
2,3-Substituted benzo[b]thiophene PfNMT and PvNMT inhibitor 1 and the target profile for the development of this series. Footnote a: Ki values are quoted in place of IC50 values as a means of expressing the inhibitor affinity while correcting for differing Michaelis constants (Km) between enzymes. Enzyme Ki values are calculated from the IC50 values using the Cheng–Prusoff equation, the definition of which is given in the Experimental Section.23 IC50 values are the mean value of two or more determinations, and standard deviation is within 20% of the IC50. Footnote b: No significant difference in inhibition between HsNMT1 and HsNMT2 isoforms has been observed in this series; therefore, the HsNMT affinities reported in this work refer to HsNMT1. Footnote c: LELP = cLogP/LE. LE = [−log(Ki)](1.374)/(no. of heavy atoms), with cLogP determined with ChemAxon, which can be obtained from http://www.chemaxon.com/products/calculator-plugins/logp/.
