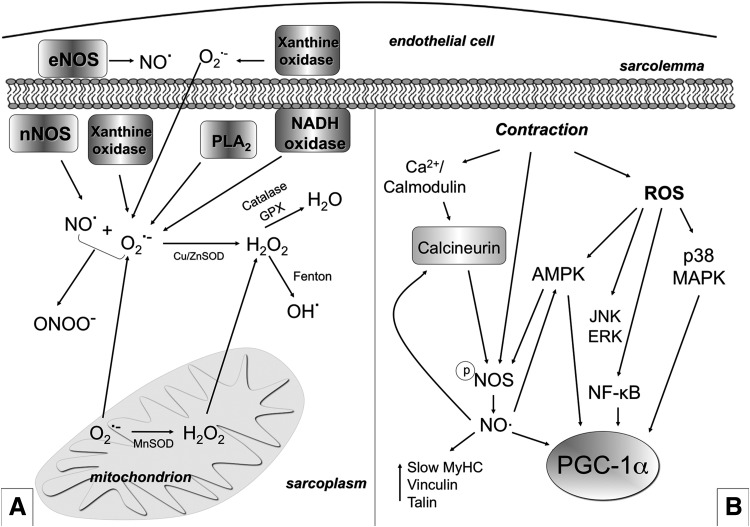
FIG. 7.
ROS and RNS production and their role in exercise-mediated skeletal muscle plasticity. (A) Several ROS and RNS are produced in skeletal muscle both at rest and during contraction. NO• is produced by NOS (eNOS in the endothelial cells and nNOS in the muscle fibers). The O2•− is generated into mitochondria in at least five sites (three of which being well characterized) within the mitochondrial respiratory chain through incomplete reduction of oxygen in the electron transport system. O2•− can also be generated as a specific product of some enzymes into mitochondria. Moreover, NADH oxidase, xanthine oxidase, and PLA2 are other O2•− generators. O2•− can undergo spontaneous dismutation or dismutation catalyzed by MnSOD (in the matrix) and Cu/ZnSOD (in the intermembrane space and in the cytosol) and form H2O2. H2O2 is cytotoxic; however, it is poorly reactive and is considered a relatively weak oxidizing agent. H2O2 is unable to oxidize DNA or lipids directly, but it can inactivate some enzymes. H2O2 might be de-tossificated in H2O by catalase or GPX or be transformed, through the Fenton reaction, into OH• which is, by contrast, highly reactive and able to damage most macromolecules, including DNA, proteins, and lipids. O2•− can react with NO• and produce ONOO−. ONOO− is able to rapidly cross membranes and is a strong oxidant agent that can lead to DNA damage and nitration of proteins. Sub-intracellular measurements and detection of ROS are usually prone to artifacts, while O2•− intracellular catabolism is extremely complex and controversial. Therefore, the earlier description is an oversimplification. Skeletal muscle has a well-developed system that prevents potentially deleterious effects of ROS (such as catalases, SODs, glutathione, GPX, peroxiredoxins, and thioredoxins). The abundance of scavengers abrogates free-radical chain reaction propagation under physiological conditions. If redox homeostasis is disrupted, the cell becomes damaged, thus leading to a pathological condition. (B) ROS and NO• mediate a contraction-induced adaptive response to exercise. NO• and ROS mediate the up-regulation of PGC-1α, GLUT4, mitochondrial genes, and slow genes. Moreover, ROS increase Glu uptake by triggering p38 MAPK (and also ERK) phosphorylation and activation, which possibly phosphorylates and activates PGC-1α. ROS and RNS trigger PGC-1α phosphorylation and Glu uptake also through AMPK. Moreover, ROS induce NF-κB-mediated transcription of PGC-α. Contraction also induces NO• production by NOS. NO•, in turn, activates calcineurin/NFAT induction of fast-to-slow phenotype. NOS is a Ca2+/calmodulin-dependent enzyme that should be dephosphorylated in order to produce NO•, and this might depend on calcineurin. NOS is also regulated by AMPK. NO•, nitric oxide; O2•−, superoxide anion; H2O2, hydrogen peroxide; GPX, glutatione peroxidase; NF-κB, nuclear factor-kappa B; NOS, nitric oxide synthases; OH•, hydroxyl radical; ONOO−, peroxynitrite; PLA2, phospholipase A2; RNS, reactive nitrogen species; SOD, superoxide dismutase.