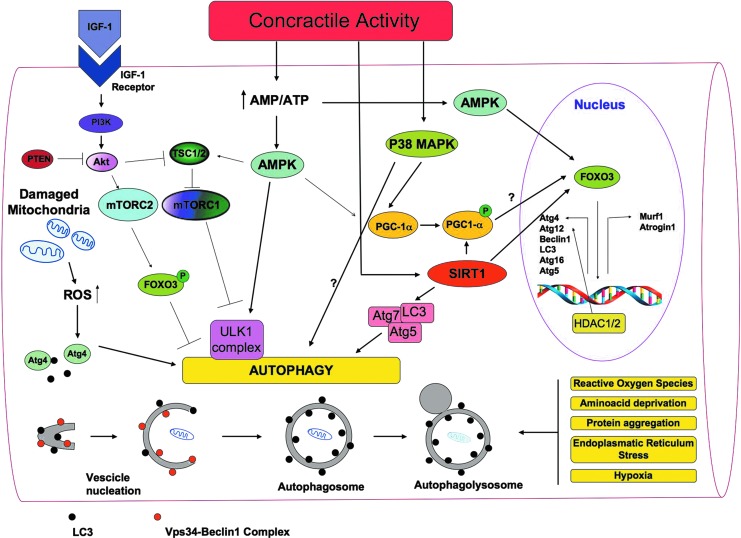
FIG. 8.
A general overview of the signaling molecules involved in the regulation of autophagy in skeletal muscles. Autophagy is a multi-step process that involves distinct phases during which part of the cytoplasm intracellular organelles are sequestered within characteristic double-membraned autophagic vacuoles (autophagosomes) that fuse with lysosomes and become autophagolysosomes. There, defective intracellular organelles and proteins are digested by a battery of lysosomal hydrolases. LC3 and the Vps34-Beclin1 complex are required, among many other proteins, for autophagosome formation. In skeletal muscles, ROS production influences different cell signaling pathways, including selective mitochondrial autophagy (mitophagy). Damaged mitochondria removal is particularly needed during exercise when the oxidative metabolism and the turnover of mitochondria increase. Moreover, ROS are mainly produced by mitochondria. The activation of the pivotal autophagy protein LC3 is mediated by the redox-sensitive Atg4 protease, which cleaves LC3. Growth factors such as IGF induce the PI3K/Akt signaling, which activates mTORC1 and mTORC2. mTORC1 (known to activate protein synthesis) also inhibits autophagy, as it inhibits the formation of the Atg1 (in humans ULK1) complex. ULK1 is a serine/threonine kinase that forms a complex with different regulatory proteins such as Atg13 and Atg17. Atg13 hyper-phosphorylation inhibits its association with Atg1, while the Atg1–Atg13 interaction enables the generation of autophagosomes. mTORC2 phosphorylates and inhibits FoxO transcription factors, thus inhibiting autophagy. Indeed, dephosphorylated FoxOs migrate into the nucleus and activate the transcription of genes that control muscle mass. FoxOs activate both ubiquitin-proteasome genes (atrogin-1 and MuRF-1) and autophagy-lysosome genes such as Beclin1, LC3, Atg4, Atg12, Atg16, and Atg5. PTEN inhibits the PI3K/Akt signaling pathway, and, therefore, it enables autophagy. Exercise, mitochondrial dysfunctions, starvation, and oxidative stress increase the intracellular AMP/ATP ratio, thus activating the energy stress sensor AMPK, which, in turn, promotes autophagy by inhibiting mTORC1 through the phosphorylation of TSC2. The TSC complex, consisting of TSC1 and TSC2 proteins, regulates the activity of the mTORC via Rheb, a small GTPase. In addition, AMPK induces autophagy by triggering ULK1 phosphorylation. By integrating signals from upstream sensors such as mTOR and AMPK, the ULK1 complex plays a central role in autophagy. AMPK activates FoxO transcription factors and leads to the expression of LC3 and Beclin1. Through a mechanism not yet known, p38 MAPK also seems to induce autophagy. In addition, SIRT1 can deacetylate Atg5, Atg7, and LC3, thus inducing autophagy; nuclear SIRT1 might induce the expression of autophagy genes through the activation of FoxOs. HDAC1 and 2 regulate muscle autophagy by controlling the expression of autophagy genes. During exercise, PGC-1α induction is mediated, among other things, by AMPK and p38 MAPK. Exercise up-regulates SIRT1 that removes acetyl groups from PGC-1α, enabling its translocation to the nucleus. PGC-1α induction has been associated with increased autophagy, although this hypothesis needs further investigation. IGF, insulin growth factor; LC3, microtubule-associated protein 1-light chain 3; mTOR, mammalian target of rapamycin; mTORC, mTOR complex; MuRF-1, muscle ring finger protein 1; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homologue; TSC, tuberosus sclerosis complex; ULK1, unc-51-like kinase1; Vps34, vacuolar protein sorting 34. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars