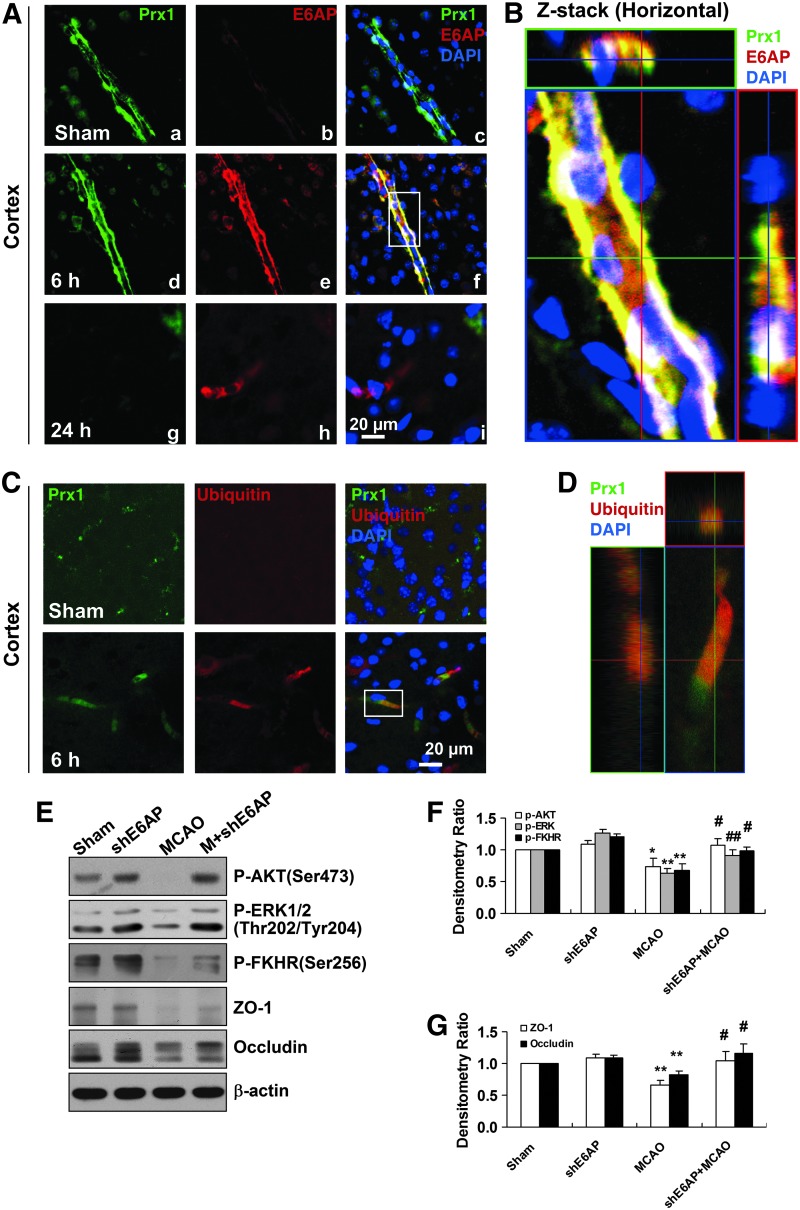
FIG. 7.
E6AP activation is associated with Prx1 ubiquitination in brain microvessels of cerebral ischemia mice. (A) Double immunohistochemical staining for Prx1 and E6AP in the penumbra after tMCAO. Fluorescence staining for Prx1 (green) and E6AP (red) was performed in ipsilateral brain regions 6 and 24 h after brain ischemic injury in mice. (B) The orthogonal projections onto the x−z (upper) and y−z (right) planes are shown to confirm the colocalization of Prx1 and E6AP throughout the microvessels shown in (A). (C) Fluorescence immunohistochemical staining of Prx1 and ubiquitin in brain microvessels. Anti-ubiquitin (red) and Prx1 (green) staining was performed 6 h after tMCAO in mice. (D) Higher-magnification image of endothelial staining from the insets is shown in (C). Each image shown is representative of five independent mice. (E) The effect of E6AP knockdown on neurovascular damage after brain ischemia in mice. The lentivirus E6AP shRNA knockdown was used to silence E6AP mRNA. The protein extracts from penumbra brain region of mice were processed for Western blotting to detect ZO-1, Occludin, and phosphorylated AKT, ERK, FHHR. (F, G) Quantitative analysis of protein levels in (E) was performed by densitometry. Densitometry values were normalized to the average of all sham values (mean±SEM, n=6). *p<0.05; **p<0.01 versus sham mice; #p<0.05; ##p<0.01 versus vehicle-treated mice. Immunoblotting with an anti-β-actin antibody demonstrated equal protein loading in each lane. tMCAO, transient middle cerebral artery occlusion; ZO-1, zonula occludens-1; shRNA, short hairpin RNA. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars