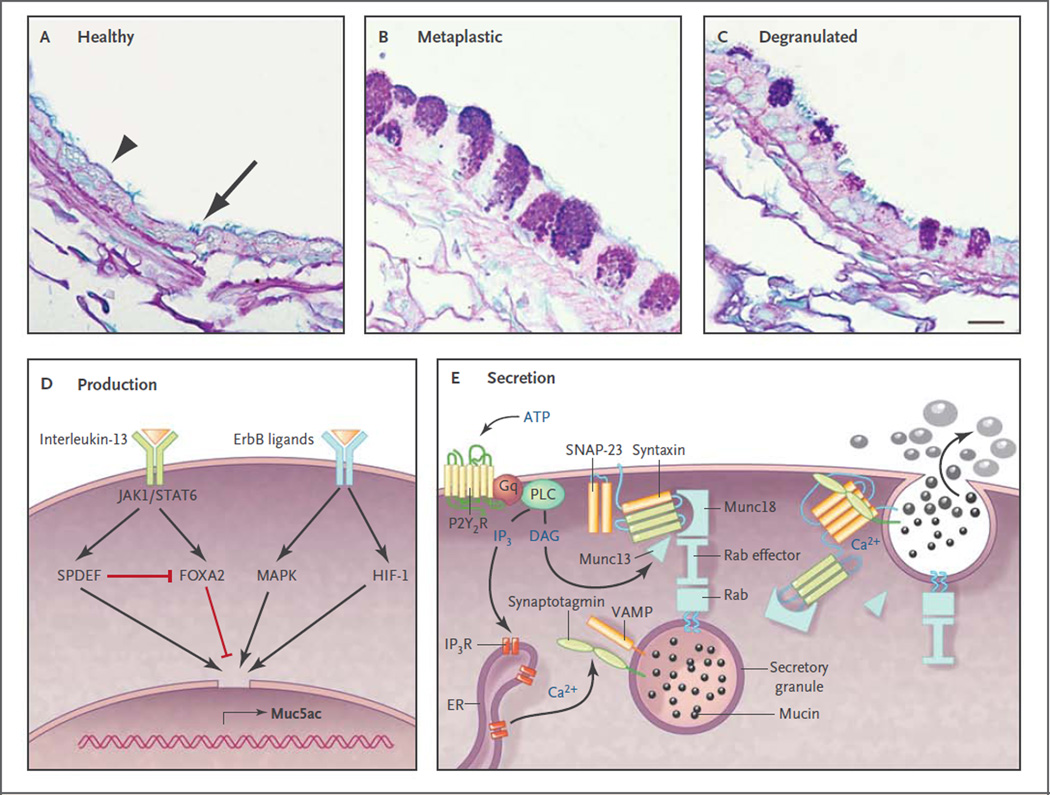
Figure 3. Production and Secretion of Polymeric Mucins.
Panels A through C show axial sections through bronchi of mice, which are similar in size and structure to human bronchioles. Panel A shows an airway under healthy conditions, in which polymeric mucin production is low so that the secretory cells (arrowhead) do not show mucin granules when stained with Alcian blue and periodic acid–Schiff reagent. Nonetheless, antibodies indicate that the cells do produce small amounts of Muc5b (not shown), although Muc5ac is undetectable.14,29 Ciliated cells are interspersed among the secretory cells (arrow). Panel B shows an airway 2 days after the induction of mucous metaplasia by asthmalike allergic inflammation due to sensitization and challenge with ovalbumin.11 Mucin-containing granules are visible in the secretory cells as a result of greatly increased Muc5ac and moderately increased Muc5b production.14,29,30 Panel C shows a metaplastic airway 10 minutes after stimulation of mucin secretion by an ATP aerosol (scale bar, 10 µm). Panel D shows ligands and transcription factors that are important in Muc5ac expression. Interleukin-13 binds to a receptor that includes the interleukin-4Rα subunit, activating Janus kinase 1 (Jak1), leading to the phosphorylation of Stat6. There is no consensus Stat6 binding site in the MUC5AC and Muc5ac promoter, but Stat6 activation leads to increased expression of SPDEF (SAM pointed domain-containing Ets transcription factor), which up-regulates multiple genes involved in mucous metaplasia,39 and inhibits expression of Foxa2, which negatively regulates Muc5ac.45 Several ligands bind ErbB receptors, including epidermal growth factor, transforming growth factor α, amphiregulin, and neuregulin, activating mitogen-activated protein kinases (MAPK).42,46 Hypoxia-inducible factor 1 (HIF-1) also can be activated downstream of ErbB receptors, and there is a conserved HIF-1 binding site in the proximal MUC5AC and Muc5ac promoter,30 but whether this is the dominant mechanism of up-regulation by ErbB ligands is not known. Not shown are complement C3 and β2-adrenergic–receptor signaling, which amplify Muc5ac production,29,47–49 or transcription factors such as Sox2, Notch, E2f4, and Math, which primarily regulate development. Panel E shows the molecular mechanism of mucin exocytosis. A mucin-containing secretory granule is docked to the plasma membrane by the interaction of a granule-bound Rab protein with an effector protein that acts as a tether to Munc18, which binds the closed conformation of Syntaxin anchored to the plasma membrane. Secretion is triggered when ATP binds to P2Y2 purinergic receptors (P2Y2R) coupled to Gq, activating phospholipase C (PLC), which generates the second messengers diacylglycerol (DAG) and inositol triphosphate (IP3). DAG activates Munc1314 to open Syntaxin so it can form a four-helix SNARE (soluble N-ethylmaleimide–sensitive factor attachment protein receptor) complex with SNAP-23 (synaptosomal-associated protein 23) and VAMP (vesicle-associated membrane protein), drawing together the granule and plasma membranes.50 IP3 induces the release of calcium from IP3 receptors (IP3R) in the endoplasmic reticulum (ER), activating Synaptotagmin51 to induce final coiling of the SNARE complex, which results in fusion of the membranes and release of the mucins. The photomicrographs are courtesy of Dr. Michael J. Tuvim.