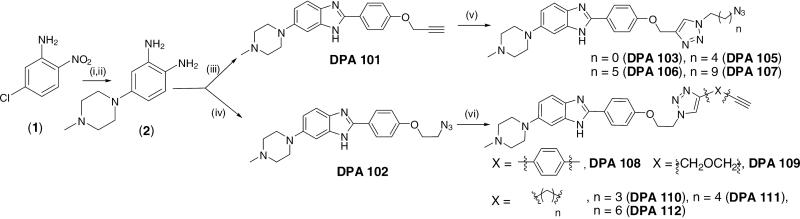
Scheme 1.
Reagents and conditions (i) DMF, N-methylpiperazine, 100 °C, 5h, 60% (ii) Pd-C (10%), ethanol, H2, 6h, qaunt. (iii) 4-(prop-2-ynyloxy) benzaldehyde (3), Na2S2O5, H2O, reflux, 12h, 65% (iv) 4-(2-azidoethoxy) benzaldehyde (4), Na2S2O5, H2O, reflux, 12h, 82% (v) sodium ascorbate, copper (II) sulfate, 50 fold excess bisazides [N3-CH2-(CH2)n-N3; n = 0(5a); n = 4(5b), n = 5(5c), n = 9(5d)], ethanol, room temperature, overnight,65-80 % (vi) sodium ascorbate, copper (II) sulfate, 50 fold excess bisalkynes [1, 4 diethynyl benzene (6a), propargyl ether (6b), HC-(CH2)n-CH; n = 3(6c), n = 4(6d), n = 6(6e)] ethanol, temperature, overnight, 75-90 %.