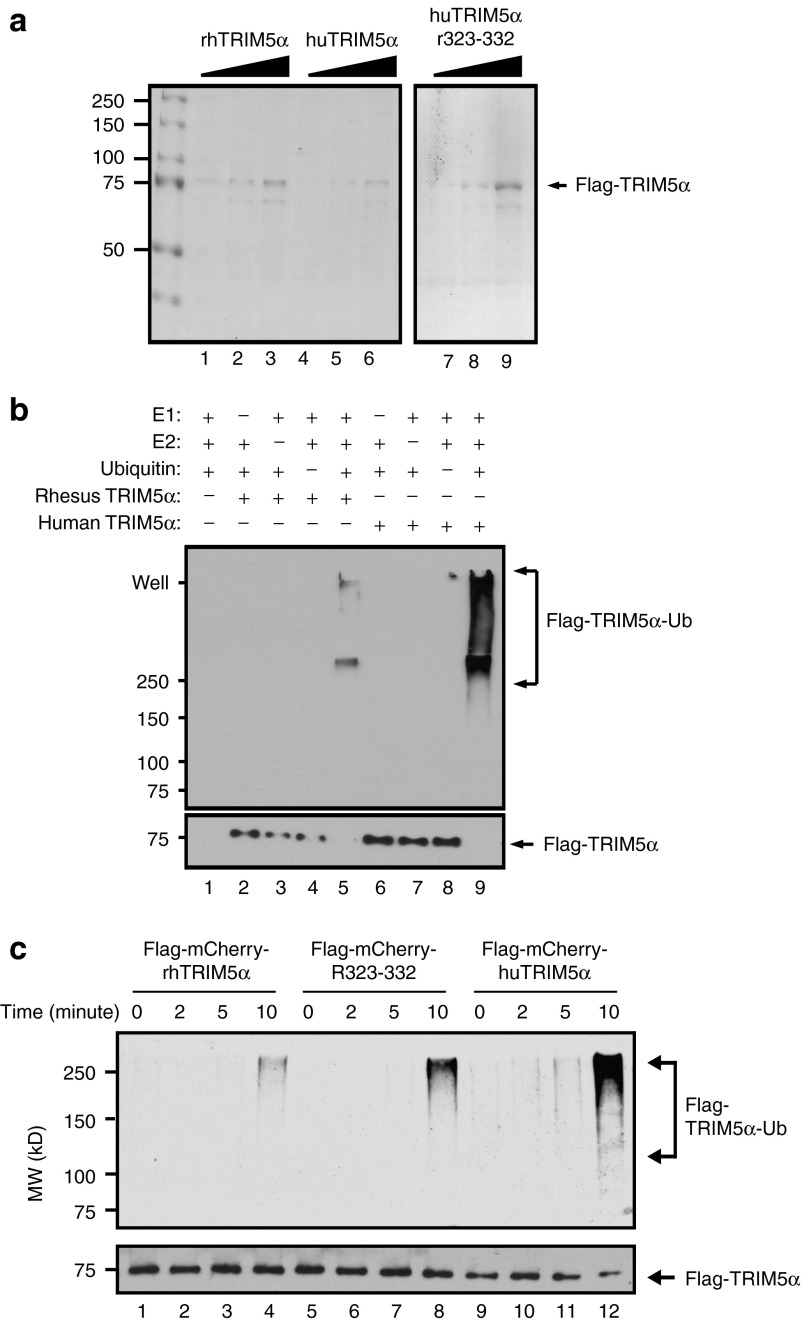
Figure 4.
HuTRIM5α has a higher propensity for self-ubiquitination than rhTRIM5α or huTRIM5αR323–332. (a) Flag-mCherry-TRIM5α proteins (rhTRIM5α, huTRIM5αR323–332, and huTRIM5α) were purified from lysates of transfected HEK293T cells by anti-Flag affinity chromatography. One, three, and ten microliters of purified lysate were analyzed on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gel: lanes 1–3 rhTRIM5α, lanes 4–6 huTRIM5α, lanes 7–9 hTRIM5αR323–332. (b) The specificity of the autoubiquitination assay was then confirmed by removing one or more reaction components (indicated by the + and – signs) from the assay, using purified huTRIM5α and rhTRIM5α separately as the E3 ligase. After 10 minutes incubation, samples were boiled in SDS–PAGE sample buffer and analyzed by western blot, using anti-ubiquitin and anti-Flag antibodies. (c) To compare the propensity of the three TRIM5α isoforms to self-ubiquitinate, purified Flag-mCherry-TRIM5α proteins (rhTRIM5α, huTRIM5αR323–332, and huTRIM5α) were incubated with ubquitination reaction components for the indicated time, and subjected to SDS–PAGE and western blotting as above. The amount of ubiquitinated Flag-TRIM5α proteins (top panel) and unmodified Flag-mCherry-TRIM5α proteins (lower panel) are shown and the reaction times, antibodies, and molecular weight standards are indicated on the top, right, and left, respectively.