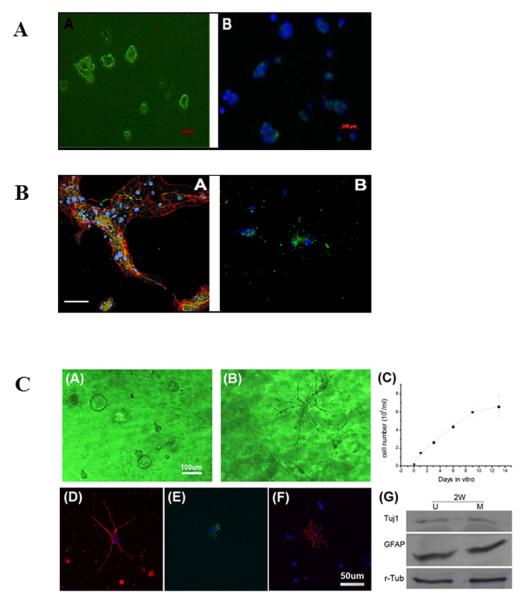
Figure 1. The bioactive scaffold UBM effectively supports the proliferation and differentiation of neural stem cells (NSCs).
(A) NSCs grown in culture. Representative images showing the cells after passage 6. NSCs were positive for nestin, an intermediate filament commonly found in neural precursors (panel A, green). Nuclei were stained with DAPI (panel B, blue); (B) Differentiated stem cells grown in culture. Representative confocal immunofluorescent images showing triple labeled differentiated NSCs after 7 days in differentiation medium (panel A); the fluorescent signal corresponds to nuclei (blue), GFAP (red), and β-tubulin-III (green). Representative confocal immunofluorescent images showing double labeled differentiated NSCs under the same treatment conditions (panel B); the fluorescent signal corresponds to nuclei (blue) and oligodendrocytic O4 (green). Scale bar: 100μm; (C) NSCs cultured in UBM (panel A) or matrigel (panel B) undergoing proliferation on day 9. UBM is better at maintaining NSCs in their naïve or undifferentiated state than matrigel.; the proliferation profile of NSCs shows a doubling time of 47.2 hours (panel C); differentiated NSCs after 7 days in differentiation medium are positive for Tuj1 (panel D), GFAP (panel E), and O4 (panel F); the differentiation of NSCs in UBM (U) and matrigel (M) was quantitatively measured by Western blot analyses (panel F) showing comparable expression of Tuj1 and GFAP. This finding suggests both neuronal and astrocytic differentiation in both matrices; r-tubulin was used as the loading control.